Introduction:
In subjects with chronic anemia syndromes, such as sickle cell disease and major thalassemia, cerebral blood flow (CBF) has been shown to increase in compensation for the decreased oxygen content. In previous works using pseudo-continuous arterial spin labeling, our laboratory has demonstrated the phenomenon of venous outflow suggestive of physiological arteriovenous shunting in anemic subjects with high CBF and short microvascular transit time (Bush et al., 2018). In this study, we evaluated CBF, oxygen delivery (DO2), oxygen extraction fraction (OEF) and cerebral metabolic rate (CMRO2) in three subject groups: sickle cell disease (SCD), non-sickle anemia (ACTL) and healthy controls (CTL). We hypothesize that even though DO2 is preserved in the presence of chronic anemia, due to physiological shunting, OEF and CMRO2 are impaired in anemic subjects compared to controls.
Methods:
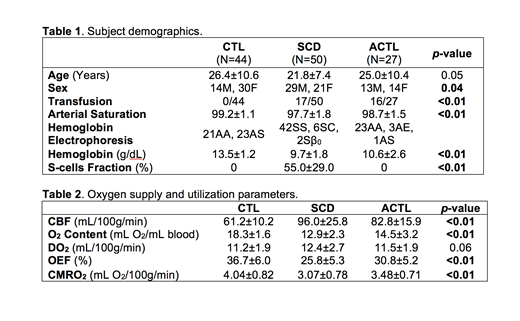
Three study groups of 50 SCD subjects, 27 ACTL subjects and 44 healthy controls were tested (Table 1). Oxygen content, DO2, OEF and CMRO2 were computed from the hemoglobin level, CBF from phase contrast MRI and venous saturation from TRUST MRI.
Results:
Table 2 shows the global DO2, OEF and CMRO2. As expected, anemic patients had approximately 50% higher CBF but similar DO2 levels compared to healthy controls. Despite normal delivery, both CMRO2 and OEF are significantly decreased in SCD and ACTL subjects.
Resting DO2, whether entered as CBF and O2 content separately or as their product, was the strongest predictor of the brain's metabolic rate, explaining approximately 21% of the variation in baseline CMRO2. Even after controlling for the variations in delivery, our subjects still demonstrated significant differences based on disease state, with the lowest CMRO2 in SCD patients, followed by ACTL and then healthy controls (p<0.01). After correcting for both DO2 and disease state, CMRO2 was independent of transfusion status, age, sex, hemolytic indices, fetal hemoglobin levels and mean corpuscular volume but remained directly proportional to hemoglobin (p=0.01) and platelets (p=0.01) with a combined r2=0.62.
Discussion:
Using a larger cohort, our results recapitulated the compensatory hyperemic response previously described in anemic subjects. Despite the ability to maintain a normal level of oxygen supply to the brain, these anemic patients had significantly lower cerebral metabolism, consistent with reports from two other laboratories (Vaclavu et al., 2019; Croal et al., 2019). We postulate that poor oxygen utilization is caused by non-nutritive perfusion or physiological arteriovenous shunting. This phenomenon has been previously characterized in SCD and other anemias with high CBF and reduced microvascular transit times. Microvascular oxygen unloading requires sufficient transit time in the capillaries network for efficient oxygen extraction, but transit times had been demonstrated to be significantly shorter in the presence of anemia due to compensatory hyperemia. In chronic anemia syndromes, the elevated CBF that preserves normal DO2 shortens transit times and impairs oxygen unloading, leading to a decrease in OEF and CMRO2 in both SCD and ACTL subjects.
The predictors of baseline CMRO2 yielded interesting insights into the physiological impact of chronic anemia on cerebral oxygen availability and utilization. In acute normovolemic anemia, CMRO2 is preserved despite declining O2 carrying capacity. However, in our chronically anemic cohort, CMRO2 was strongly proportional to resting DO2; this result suggests that there is a divergence in physiological responses to acute versus chronic anemia. While DO2 was the strongest predictor of CMRO2, disease state, hemoglobin level and platelets remained in the multivariate model, confirming the roles of anemia, inflammation, and disease-specific factors in the modulating CMRO2.
In summary, this report demonstrates impaired cerebral oxygen supply-demand matching in chronically anemic subjects. Cerebral hyperemia appears to simultaneously preserve DO2 while diminishing OEF and CMRO2. This observation may explain why absolute hemoglobin level remains the strongest predictor of poor neurovascular outcome in SCD patients, and raises questions regarding the proper hemoglobin target level for hydroxyurea and chronic transfusion therapy.
Coates:celgene: Consultancy, Honoraria, Other: steering committee of clinical study; vifor: Consultancy, Honoraria; agios pharma: Consultancy, Honoraria; apo pharma: Consultancy, Honoraria, Speakers Bureau. Wood:National Institutes of Health: Research Funding; Philips Healthcare: Research Funding; BluebirdBio: Consultancy; Celgene: Consultancy; BiomedInformatics: Consultancy; Imago Biosciences: Consultancy; Apopharma: Consultancy; WorldcareClinical: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal