Introduction:
Many patients with newly diagnosed acute myeloid leukemia (AML) or myelodysplastic syndrome with 10-19% blasts (MDS-EB2) do not enter complete remission (CR) following initial induction chemotherapy. At an academic referral center, such patients often stay to receive additional treatment, or return to their home communities for further care. For patients and providers alike, the decision about whether to stay or go after initial treatment failure is often fraught. To better inform such decision-making, in this retrospective single-center analysis, we compared covariate-adjusted survival for patients who elected to stay for further treatment at our center and those who returned to their home communities for subsequent care.
Methods:
We included adults ≥ age 18 years of age with newly-diagnosed AML or MDS-EB2 treated at our institution between January 2012 and May 2018 who failed to enter CR (< 5% morphologic bone marrow blasts) or CR with incomplete hematologic recovery (CRi) after their first cycle of induction chemotherapy. We excluded patients who died before they could begin re-induction therapy. Patients who stayed at our institution for additional treatment are referred to as the "stay" group (n=86); patients who left are considered the "go" group (n=35). Multivariable Cox regression analysis was used to account for other measured covariates possibly influencing survival.
Results:
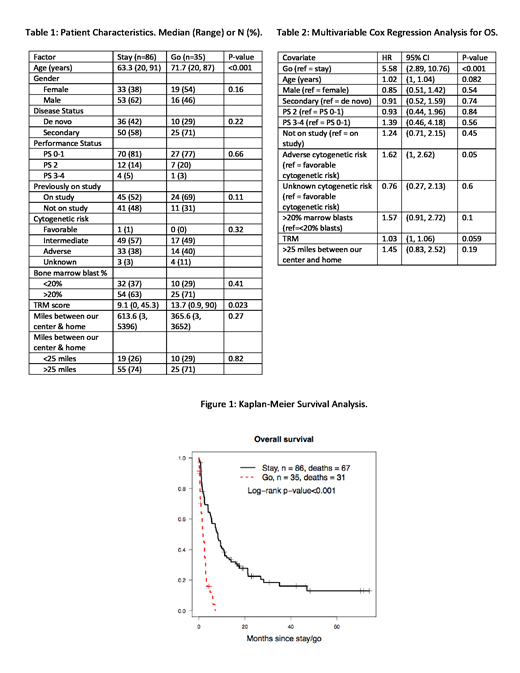
The go group was older and had a higher median treatment-related mortality (TRM) score (Table 1), the latter predictive of the probability of death within the first 28 days of initial induction therapy. Forty-seven percent of stay patients received high-intensity re-induction (containing cytarabine at individual doses ≥1g/m2) while 50% received low-intensity treatment (e.g. azacitidine, decitabine, or low-dose cytarabine). Twenty-nine percent of go patients received treatment (mostly low-intensity) in the community setting, while 63% received supportive care only. The stay patients had a median of 2 subsequent hospitalizations (range 0-12) and spent a median of 27 days hospitalized after initial treatment failure (range 0-124). Survival was longer in the stay group compared to the go group (median 8.3 vs. 1.8 months, p<0.001, Figure 1). After accounting for covariates, the risk of death was 5.6-fold higher (95% CI 2.9-10.8, p<0.001) in go patients (Table 2). Median follow-up of patients alive at last contact was 20 months in the stay group and 1 month in the go group; 31/35 go patients have died (Figure 1). Re-induction treatment produced CR or CRi in 41/86 stay patients (48%). Achievement of CR was the covariate most strongly associated with survival in the stay group, with a time-dependent regression model for CR demonstrating a hazard ratio (HR) of 0.32 (95% CI 0.18-0.56, p<0.001); the deleterious effect of adverse cytogenetics on survival (Table 2) likely reflects its association with the inability to achieve CR. Improved survival was incompletely explained by receipt of allogeneic hematopoietic cell transplant (HCT), though there was a trend toward improved survival among the 30 patients (35%) in the stay group who underwent allogeneic HCT (time-dependent HR 0.73, 95% CI 0.41-1.3, p=0.28).
Discussion:
Patients with refractory AML or MDS-EB2 who elected to stay at our institution for additional therapy after initial treatment failure were more likely to live longer than those who returned to their communities. Those in the go group mostly received supportive care alone. Although the survival benefit among the stay group was modest (median additional survival of 6.5 months), it was similar to the incremental survival benefits associated with several recently approved drugs and was strongly associated with achievement of CR, which occurred in 48% of stay patients. Although our study is limited by the inability to account for unknown covariates, a definitive randomized study is not feasible. There is also a relative lack of information about the go patients once they left our center, although we do know that these patients were much less likely to receive AML/MDS-directed therapy than their stay counterparts. A principal problem in advising patients to stay for additional treatment is our limited ability to predict CR with re-induction therapy. However, our findings suggest that patients with non-adverse cytogenetics and lower TRM scores might preferentially be so advised (Table 2).
Othus:Celgene: Other: Data Safety and Monitoring Committee; Glycomimetics: Other: Data Safety and Monitoring Committee. Gardner:Abbvie: Speakers Bureau. Halpern:Pfizer Pharmaceuticals: Research Funding; Bayer Pharmaceuticals: Research Funding. Scott:Novartis: Consultancy; Agios: Consultancy; Incyte: Consultancy; Celgene: Consultancy. Becker:The France Foundation: Honoraria; Accordant Health Services/Caremark: Consultancy; AbbVie, Amgen, Bristol-Myers Squibb, Glycomimetics, Invivoscribe, JW Pharmaceuticals, Novartis, Trovagene: Research Funding. Percival:Pfizer Inc.: Research Funding; Nohla Therapeutics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal