Introduction: The 7 + 3 regimen, consisting of cytarabine treatment continuously for 7 days, and short infusions of an anthracycline on each of the first 3 days, has been the mainstay for adult acute myeloid leukemia (AML) therapy. However, in older adults and patients with secondary AML, 7+3 is associated with lower remission rates, shorter overall survival, and higher relapse rates than in younger adults and older patients with de novo AML (Kayser et al, Blood 117:2137-2145, 2011; Schoch et al, Leukemia 18: 120-125, 2004). CPX-351 (VYXEOS®) CPX-351 was approved by the FDA in the US in August 2017 and EU in August 2018 for the treatment of adults with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). It is a fixed combination of daunorubicin and cytarabine at a synergistic 1:5 molar ratio, encapsulated in a liposome to provide optimal delivery of the two drugs. The Phase III pivotal trial demonstrated that CPX-351 significantly improved overall survival (OS; 9.56 vs 5.95 months; hazard ratio [HR] = 0.69; 1-sided P = 0.003) and complete remission rates versus 7+3, and was associated with shorter per patient year (PPY) hospital length of stay (LOS) without an increase in PPY non-drug costs (Chung, et al. AMCP HCRU Abstract JOV-62933; 2018). This study characterized the patients treated with CPX-351 and 7+3 and compared healthcare utilization and costs associated with the treatments using a large U.S.-based hospital administrative database.
Methods: A retrospective, observational study was conducted using data from the Premier Healthcare Database. The study included patients aged 18+ years with any inpatient hospitalization or hospital-based outpatient visit with a diagnosis of AML, who received treatment of 7+3 or CPX-351 between 8/1/2017 and 2/28/2019. Variable-length follow-up was used. Patients were excluded if their length of follow-up was shorter than 30 days, or they had received treatment with gemtuzumab, midostaurin, sorafenib, venetoclax, quizartinib, or gilteritinib. Patients were assigned into two cohorts: 1) patients treated with CPX-351; 2) 7+3 treated patients with newly diagnosed t-AML or AML-MRC who were never treated with CPX-351 (CPX-351 eligible 7+3). Outcomes included total inpatient LOS, total costs, drug and non-drug costs. The outcomes were converted to per patient year (PPY) values, which was the original value divided by the length of follow-up and multiplied by 365. Unadjusted descriptive statistics were reported, and bivariate analysis was performed using Wilcoxon rank sum tests to examine the difference in outcomes between 7+3 treated patients and those treated with CPX-351.
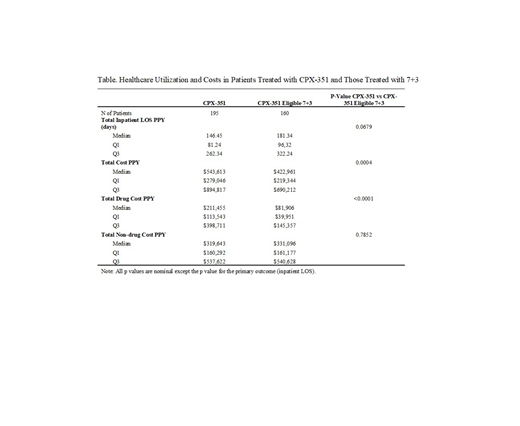
Results: The study included 195 qualifying patients treated with CPX-351, and 160 CPX-351 eligible 7+3 patients. The median age was 68 years (Q1-Q3: 61-71) in the CPX-351 patients and 61 years (Q1-Q3: 52.5-69) in CPX-351 eligible 7+3 patients. Male patients accounted for 62.6% of the CPX-351 patients and 55.0% of the CPX-351 eligible 7+3 patients. Among the CPX-351 patients, 72.3% were White, while the proportion of White is 75.0% in CPX-351 eligible patients. The median length of follow up was 136 days (Q1-Q3: 68-232) in the CPX-351 patients, and 126 days (Q1-Q3: 54.5-241.5) in the CPX-351 eligible 7+3 patients. The median total inpatient LOS per patient year (PPY) was shorter in CPX-351 patients compared to the CPX-351 eligible 7+3 patients (nominal p=0.068, Table). Although the median total hospital cost PPY of the CPX-351 patients was higher (nominal p<0.001), the non-drug medical cost PPY was not higher than the CPX-351 eligible 7+3 patients (nominal p=0.785, Table).
Conclusions: In this inferential analysis, patients treated with CPX-351 appeared to be older than those treated with 7+3. CPX-351 treated patients spent fewer days as inpatients, and the non-drug medical cost incurred was not higher compared to the 7+3 patients eligible for CPX-351, although the drug costs for the CPX-351 treated patients were higher than the 7+3 treated patients. Further adjusted analysis is warranted to control for the differences in baseline characteristics such as age, clinical conditions, and other characteristics in this real-world data.
Price:Jazz Pharmaceuticals: Employment, Equity Ownership. Cao:Jazz Pharmaceuticals: Other: Zhun Cao is an employee of Premier Inc, which receives payments from Jazz Pharmaceuticals for this research project.; Premier Inc.: Employment, Other: Premier received funding from Jazz Parmaceuticals to perform the study and analysis. Lipkin:Jazz Pharmaceuticals: Other: Craig Lipkin is an employee of Premier Inc, which receives payments from Jazz Pharmaceuticals for this research project.; Premier Inc: Employment. Robinson:Premier Inc.: Employment, Other: Premier received funding from Jazz Pharmaceuticals to perform the study and analysis; Jazz Pharmaceuticals: Other: Scott Robinson is an employee of Premier Inc, which receives payments from Jazz Pharmaceuticals for this research project.. Profant:Jazz Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal