Introduction. The diagnosis of multiple myeloma (MM) is often made in elderly individuals (median age at diagnosis 69 years) with over a third of patients exceeding 75 years of age. Many elderly patients are frequently excluded from clinical trials because of predefined upper age limit of 65 years limiting accessibility to autologous stem cell transplant (ASCT) for a large proportion of MM patients. Based on limited multicenter studies and randomized clinical trials in this population, we used the National Cancer Database (NCDB) to assess the outcomes of ASCT in MM patients older than 65 years of age.
Methods. We queried the NCDB for patients with MM diagnosed between 2004 -2015 treated with ASCT as frontline therapy, yielding a final cohort of 9,383 patients. Multivariable logistic regression was used to determine the likelihood of receiving ASCT. Overall survival (OS) was calculated from the date of diagnosis to the date of last contact or death using Kaplan Meier methodology. Adjusted hazard ratios (HR) and 95% confidence interval (CI) are reported, with α=0.05 used to indicate statistical significance.
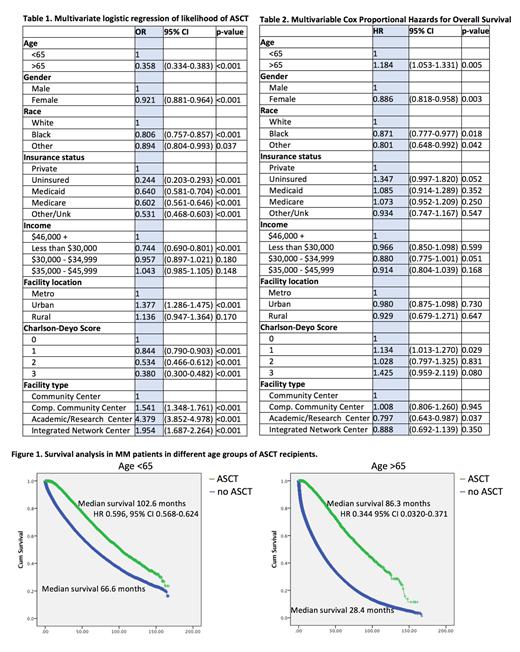
Results. The median age was 67.0 (range: 19-90). The majority of patients were older than 65 (58.5%), male (54.7%), Caucasian (75.6%), had government insurance (88%)and had a Charlson-Deyo Comorbidity score (CDCS) of 0 (75.5%). In addition to older age, insurance status, low annual income higher CDCS were associated with lower chances of receiving ASCT (Table 1). Patients in Urban areas (OR 1.377, CI 95% 1.286-1.475), and patients in Academic/Research Centers (OR 4.379 were CI 95%, 3.852-4.978) were more likely to receive ASCT. In patients younger than 65-years of age rate of ASCT was 7.0% in 2004 and increased to 16.7% in 2015. The annual rate of ASCT in patients older than 65-years of age was 1.1% in 2004 and increased to 4.7% in 2015. The multivariable cox proportional hazards of death in patients receiving ASCT was associated with improved survival (HR 0.492, CI 95% 0.473-0.512). Age (>65 years old HR 1.184, 95% CI 1.053-1.331), insurance status (uninsured HR 1.361, CI 95% 1.302-1.422, Medicaid HR 1.365, 95% CI 1.318-1.414, Medicare HR 1.271, 95% CI 1.244-1.299) and higher comorbidity score were associated with worse survival (Table 2). Median survival in patients younger than 65-years old receiving ASCT was 102.6 month versus 66.6 months in patients not receiving ASCT (HR 0.596, 95% CI 0.568-0.624) (Figure 1). Median survival in patients older than 65 years old and not receiving ASCT was 86.3 months versus 28.4 months in patients not receiving ASCT (HR 0.344, 95% CI 0.0320-0.371).
Conclusions. The findings of the present study demonstrate decreased utilization of ASCT in older patients with MM, despite significant survival benefit of such therapy. Other factors associated with decreased likelihood of ASCT are such as insurance status and annual income unfold existing disparities in patients with MM receiving ASCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal