Background: Immune thrombotic thrombocytopenic purpura (iTTP) is a potentially fatal syndrome, resulting primarily from autoantibodies against ADAMTS13. However, the mechanism underlying the autoantibody formation and the contribution of other genomic alterations to the pathogenesis of iTTP are largely unknown.
Methods: Whole exome sequencing (WES) and bioinformatic analyses were performed to determine the genetic variations in 40 patients with iTTP who had ADAMTS13 activity <10 IU/dL and a positive inhibitor or an elevated anti-ADAMTS13 IgG in concordance with clinical presentations of severe thrombocytopenia and microangiopathic hemolytic anemia with various degrees of organ injury. WES was also performed at the same time in fifteen age-, gender-, and ethnicity- matched individuals who did not have a history of iTTP or other hematological disorders as controls.
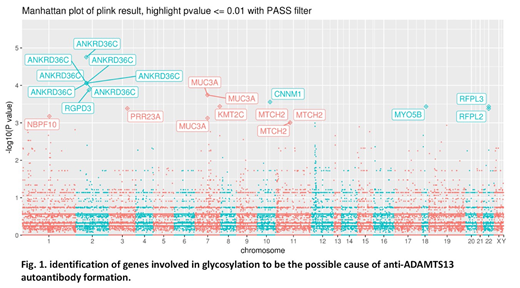
Results: WES identified variants or mutations in the genes involving in glycosylation, including O-linked glycosylation, to be the major pathway affected in patients with iTTP. We propose that the altered glycosylation may be responsible for the development of autoantibodies against ADAMTS13 which impair the proteolytic cleavage of von Willebrand factor, accelerate the clearance of ADAMTS13 from circulation, and result in severe thrombocytopenia platelets in patients with iTTP. We also identified defects in ankyrin repeat containing protein ANKRD36C, a protein with hitherto unknown function, as the most statistically significant genomic alterations associated with iTTP (p < 10-5). Moreover, candidate gene analysis revealed that various genes involving in hemostasis, complement activation, platelet function and signaling pathway, and inflammation were all affected in patients with iTTP, which may contribute to the onset, progress, severity, and long-term outcome of iTTP. Finally, we also identified two patient subgroups where the disease mechanism might be different.
Conclusion: Our findings provide novel insight into the pathogenic mechanism underlying ADAMTS13 autoantibody production and the potential contribution of other genetic abnormalities in modifying the iTTP clinical presentations in the individuals with severe deficiency of plasma ADAMTS13 activity.
Zheng:Alexion: Speakers Bureau; Ablynx/Sanofi: Consultancy, Speakers Bureau; Shire/Takeda: Research Funding; Clotsolution: Other: Co-Founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal