Introduction:
Current guidelines recommend low-molecular-weight heparins (LMWH) over Vitamin K Antagonists (VKA) or Non-Vitamin K Antagonist Oral Anticoagulants (NOAC) for the treatment of cancer-associated venous thromboembolism (CAT), but also highlight that ultimately the choice of anticoagulant depends on patient-specific factors such as patient preferences, which is important for the acceptance of the treatment and, thus, for adherence and persistence.
So far, little is known about the specific preferences of patients with CAT with respect to anticoagulation therapy. More specifically, the impact of dosing regimen, convenience and costs on patient preferences in CAT is poorly understood. Therefore, the objective of this discrete choice experiment (DCE) was to elucidate patient preferences regarding anticoagulation convenience attributes.
Methods:
Adult patients with active cancer who experienced a CAT event and for whom the decision was made to start a treatment with rivaroxaban after being treated with the standard of care anticoagulation (LMWH/VKA) for at least four weeks were included in a multinational, observational, single-arm study (COSIMO). As part of this study, a DCE was presented to the participants, who were asked to decide between complete hypothetical treatment options based on a combination of different attributes, regardless of efficacy or safety. The following attributes were preselected in a face-to-face discussion with three focus patients and in-depth interviews with four additional patients:
route of administration (injection / tablet),
frequency of intake (once / twice daily),
need of regular controls of the International Normalized Ratio (INR) at least every 3-4 weeks (yes/no),
interactions with food/alcohol (yes/no).
Additionally, distance to treating physician (1 km vs. 20 km) was included as neutral comparator to express patients' overall utility in terms of a comprehensible unit. The relative importance of treatment attributes in terms of distances were calculated based on ratios between the utility estimates for each attribute.
A fractional factorial design was generated resulting in nine hypothetical choice sets, supplemented by a test choice set to assess the consistency of a patient's responses.
DCE data was collected by semi-structured telephone interviews, performed between week 4 and week 12 after enrollment of patients in the study and start of rivaroxaban. For each patient participating in the DCE interview, a written informed consent was obtained.
Patient preferences were analyzed based on a conditional logit regression model.
Results:
Overall, 163 patients were included (Europe: 119; Canada: 41; Australia: 3), mean age 63.7 years, 49.1% were females and diagnosed with cancer for on average 22.4 months. Most patients in the COSIMO study changed to rivaroxaban from LMWH (> 95.0 %). The median time from diagnosis of index CAT event to conduct of DCE was 150 days (IQR 88-229).
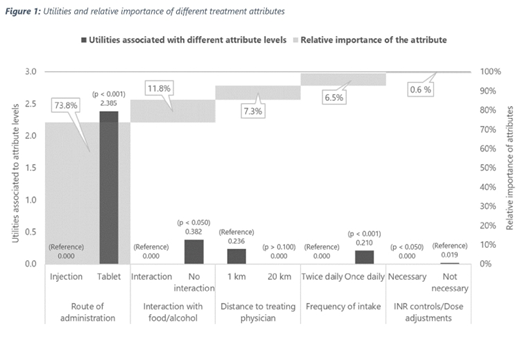
Patients strongly preferred oral administration compared to self-injections and drugs that can be taken irrespective of type of food or alcohol consumption (Figure 1). Furthermore, patients indicated slight preference for a shorter distance to the treating physician and a once daily dosing regimen compared to a twice-daily intake. The attribute "INR controls" showed no significant impact on the treatment decision.
In order of patients' preference for their choice of treatment, the route of administration was by far the most important attribute for a patient's choice (73.8% of the overall decision), followed by food interactions (11.8%), the distance to treating physician (7.2%) and the intake frequency (6.5%).
Accordingly, the expected utility of patients receiving an oral anticoagulation can be expressed as willingness to travel an additional distance of 192 km to the treating physician in order to avoid an injection.
Conclusions
Treatment related decision-making of patients with CAT, assuming equal effectiveness and safety of treatments, is predominantly driven by "route of administration", indicating a strong preference for oral intake.
Picker:Ingress-Health: Employment. Cohen:Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ACI Clinical: Consultancy; GLG: Consultancy; GlaxoSmithKline: Consultancy, Speakers Bureau; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Consultancy; Boston Scientific: Consultancy; AbbVie: Consultancy; Boehringer-Ingelheim: Consultancy, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Aspen: Consultancy, Speakers Bureau; Guidepoint Global: Consultancy; Johnson and Johnson: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Leo Pharma: Consultancy; Medscape: Consultancy, Speakers Bureau; McKinsey: Consultancy; Navigant: Consultancy; ONO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy; Temasek Capital: Consultancy; TRN: Consultancy; UK Government Health Select Committee: Other: advised the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the NHS, on the prevention of VTE; Lifeblood: Other: advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism. Maraveyas:Bayer AG: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Pfizer: Honoraria. Beyer-Westendorf:Pfizer: Honoraria, Research Funding; Bayer HealthCare: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding. Lee:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria; LEO Pharma: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Mantovani:Fondazione Charta: Consultancy; Bayer AG: Honoraria; Boehringer Ingelheim: Honoraria, Research Funding; Pfizer: Honoraria; Daiichi Sankyo: Research Funding. De Sanctis:Bayer US LLC: Employment, Equity Ownership. Abdelgawwad:Bayer AG: Employment. Fatoba:Bayer AG: Employment. Bach:Bayer AG: Employment. Wilke:Astra Zeneca: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Pharmerit: Consultancy, Honoraria; Bayer AG: Consultancy, Honoraria; LEO Pharma: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Boehringer Ingelheim: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal