Background: Randomized controlled trials leading to the approval of apixaban and rivaroxaban for venous thromboembolism (VTE) did not include patients with UE-DVT. Limited observational data has been published on outcomes in this setting and no direct comparison to traditional anticoagulation has been performed.
Objective: Evaluate the safety and effectiveness of rivaroxaban and apixaban for the treatment of acute UE-DVT compared to low-molecular weight (LWMH) and warfarin.
Methods: Consecutive patients with VTE were enrolled into the Mayo Clinic VTE Registry, between March 1, 2013 and July 22, 2019, were followed prospectively. Clinical, demographic and imaging data were collected at the time of study recruitment. Patients with a diagnosis of acute UE-DVT who received either rivaroxaban, apixaban, LMWH or warfarin within the first 14 days after diagnosis and who completed at least 3 months of anticoagulant therapy or had an outcome event in that period were included into the analysis. Recurrent VTE, major bleeding, clinical relevant non-major bleeding (CRNMB), and death were assessed at 3 months, in person whenever feasible, or by mailing a written questionnaire or a scripted phone interview. The study was approved by Mayo Clinic Institutional Review Board.
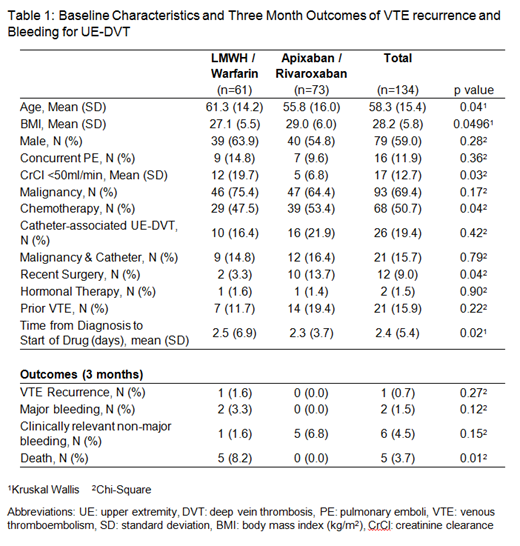
Results: One hundred and thirty-four patients with acute UE-DVT were included. Forty-one were treated with apixaban, 32 with rivaroxaban, and 61 with LWMH and/or warfarin. The mean age was 58.3 years, mean BMI was 28.2, and 59% were male (Table 1). Patients treated with LMWH / warfarin were older (61.3 vs 55.8, p=0.04) and had a lower BMI (27.1 vs 29.0, p=0.0496). Twenty-six (19%) patients had catheter-associated UE-DVT, 93 (69.4%) had a diagnosis of malignancy, and 16 (11.9%) had concurrent pulmonary embolism, which were similar between groups. More patients on apixaban /rivaroxaban were receiving chemotherapy (53.4% vs 47.5%, p=0.04) and had recent surgery (13.7% vs 3.3%, p=0.04) compared to LMWH / warfarin. At 3 months of follow up, 1 recurrent VTE occurred in patients treated with LMWH /warfarin and none of those treated with apixaban /rivaroxaban (p=0.27). Major bleeding occurred in 2 patients treated with LMWH /warfarin and in none of those treated with apixaban /rivaroxaban (p=0.12). CRNMB occurred in 1 patient treated with LWMH /warfarin and 5 patients treated with apixaban / rivaroxaban (p=0.15). Two of the CRNMBs occurred with rivaroxaban and 3 occurred with apixaban. Death occurred in 5 patients treated with LMWH /warfarin and in none of the apixaban /rivaroxaban treated patients (p=0.01).
Conclusion: This single institution prospective study adds to the limited experience with apixaban and rivaroxaban for acute UE-DVT. Treatment of UE-DVT with rivaroxaban and apixaban appears to be as safe and effective as LMWH/warfarin after 3 months of anticoagulation.
McBane:BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal