Background. Despite the improvement of therapeutic regimens, a relevant proportion of multiple myeloma (MM) patients (pts) experience early relapse (ER) [Majithia, Leukemia 2016] and represents an unmet medical need. It is therefore of high clinical interest to identify baseline factors that may predict ER.
Aims
To design models predictive of ER (defined as pts with a time-to-progression ≤18 or ≤ 24 months).
To assess the accuracy of every model on an independent validation set.
To build a score to predict ER.
Methods
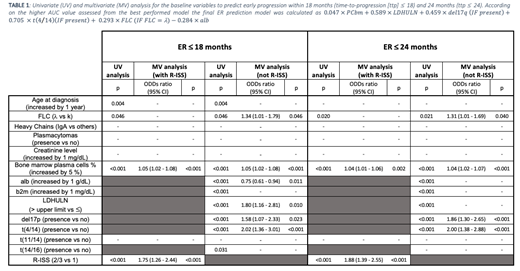
Data were obtained from 2326 pts enrolled in 8 multi-center clinical trials: NCT01093196, NCT01346787, NCT01857115, NCT01190787, NCT00551928, NCT01091831, NCT01063179 and 2005-004730-41. Here, we included 14 baseline features (fts): age, creatinine, albumin (alb), b2microglobulin (b2m), bone marrow plasma cell (PCbm) were evaluated as continuous variables; free light chain (FLC, λ vs K), M-component subtype (IgA vs others), Revised International Staging System (R-ISS stage II/III vs I), lactate dehydrogenase levels >/≤ upper limit of normal (LDHULN), presence vs absence of chromosomal abnormalities detected by FISH [del17p, t(4;14), t(14;16), t(11;14)], and presence of plasmacytomas as categorical values.
Trials were assigned to training and validation set to have a superimposed median (μe) age and follow-up in the two subsets. From the training set, a univariate analysis (UV) on outcome was performed according to both Chi-square and Kruskal tests, as appropriate. Features with p<0.05 were then tested in a multivariate (MV) logistic regression model. Each MV model was based on pts with complete data available. We performed and compared 2 MV models, one including R-ISS and one including fts (LDHULN, alb, b2m and chromosomal abnormalities) defining the R-ISS considered individually. Secondly, each model was tested on the validation set assessing the area under the curve (AUC). From the most accurate model, a score defined low (L), intermediate (I) and high (H) chances of ER. Statistical analysis was performed via R (v.3.5.2).
Results
ER≤18 models. 10/14 fts were selected based on UV analysis: age, FLC, PCbm, del17p, t(4/14), t(14/16), alb and b2m, LDHULN and R-ISS stage. Pts with complete data were included in the training set (n=923; μe age =66 years [y]; ER=35%) and the validation set (n=313; μe age=67 y; ER=36%), respectively. In the MV incorporating the R-ISS, the R-ISS II/III vs I (OR=1.75, 95% CI:1.26-2.44) and increased PCbm (OR=1.05, 95% CI:1.02-1.08) increased the risk of ER. When the MV analysis was performed including single fts instead of R-ISS, increased PCbm (OR=1.05, 95% CI:1.02-1.08), λ FLC (OR=1.34, 95% CI:1.01-1.79), LDHULN (OR=1.80, 95% CI:1.16-2.81), presence of both del17q (OR=1.58, 95% CI:1.07-2.33) and t(4/14) (OR=2.01, 95% CI:1.36-3.01) increased the probability of ER; increased alb (OR=0.75, 95% CI:0.61-0.94), reduced the risk of ER (table 1).
ER≤24 models. 8/14 fts were selected based on UV analysis: FLC, PCbm, del17p, t(4/14), alb and b2m levels, LDHULN and R-ISS stage. Pts with complete data were included in the training set (n=1009; μe age=67 y; ER=45%) and in the validation set (n=352; μe age=67 y, ER=45%). In MV analysis, including R-ISS, both R-ISS (OR=1.88, 95% CI:1.39-2.55) and PCbm (OR=1.04, 95% CI:1.02-1.06) impacted on outcome. When the MV analysis was performed including single fts, λ FLC (OR=1.31, 95% CI:1.01-1.69), PCbm (OR=1.04, 95% CI:1.02-1.07), del17p (OR=1.86, 95% CI:1.30-2.65) and t(4/14) (OR=2.00, 95% CI:1.38-2.88) retained their impact on outcome (table 1).
Validation. Each MV model was tested on the validation set. Among the 4 MV models, the ER≤18 incorporating individual fts resulted in the highest AUC (0.66) and was therefore used to build up a prognostic score.
Score. The ER score was calculated as 0.047 × PCbm + 0.589 × LDHULN + 0.459 × del17q (IF present) + 0.705 × t(4/14) (IF present) + 0.293 × FLC (IF FLC =λ) - 0.284 × alb. The ER score was calculated as 3 groups of pts with different risks of ER: L (42% of pts; risk of ER18=23%), M (33% of pts; risk of ER18=39%) and H (26% of pts; risk of ER18 =55%).
Discussion. This is the first analysis proposing a score that includes standard baseline fts and aims at identifying pts at high risk of ER in the context of novel agent-based therapy. Based on our score, 26% of pts can be defined at high risk. To improve the clinical applicability, the construction of a simplified model with categorized variables is ongoing.
Petrucci:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Offidani:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Di Raimondo:Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy. Liberati:Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial support; Roche: Other: Clinical trial support; Novartis: Other: Clinical trial support; Janssen: Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial support; Celgene: Honoraria, Other: Clinical trial support; Bristol-Myers Squibb: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy. Omedé:Janssen: Membership on an entity's Board of Directors or advisory committees. Mannina:Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Caravita di Toritto:Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accomodation costs, Research Funding; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accomodation costs; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accomodation costs; Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Patriarca:Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Benevolo:Novartis Pharmaceuticals: Consultancy. Belotti:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Gaidano:AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra-Zeneca: Consultancy, Honoraria; Sunesys: Consultancy, Honoraria. Hajek:Amgen: Honoraria, Other: Consultant or advisory relationship, Research Funding; Celgene: Honoraria, Other: Consultant or advisory relationship, Research Funding; AbbVie: Other: Consultant or advisory relationship; Bristol-Myers Squibb: Honoraria, Other: Consultant or advisory relationship, Research Funding; Novartis: Other: Consultant or advisory relationship, Research Funding; PharmaMar: Honoraria, Other: Consultant or advisory relationship; Takeda: Honoraria, Other: Consultant or advisory relationship, Research Funding; Janssen: Honoraria, Other: Consultant or advisory relationship, Research Funding. Spencer:Sanofi: Other: Consulting/advisory role; Specialised Therapeutics Australia: Consultancy, Honoraria; Amgen: Other: Consulting/advisory role, Research Funding; AbbVie: Other: Consulting/advisory role, Research Funding; Haemalogix: Other: Consulting/advisory role; Janssen Oncology: Other: Consulting/advisory role, Research Funding, Speakers Bureau; Servier: Other: Consulting/advisory role; Secura Bio: Other: Consulting/advisory role; Takeda: Other: Consulting/advisory role, Research Funding; Celgene: Other: Consulting/advisory role, Research Funding, Speakers Bureau. Sonneveld:Amgen: Honoraria, Research Funding; BMS: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; SkylineDx: Research Funding. Boccadoro:Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Mundipharma: Research Funding; Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Gay:AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.
The presentation includes discussion of off-label use of a drug or drugs for the treatment of multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal