Background
LentiGlobin for SCD gene therapy contains autologous CD34+ hematopoietic stem cells (HSCs) transduced with the BB305 lentiviral vector (LVV) encoding β-globin with an anti-sickling substitution (T87Q). Its safety and efficacy are being studied in the ongoing multi-center Phase 1/2 HGB-206 trial (NCT02140554). Initial patients received LentiGlobin drug product (DP) using bone marrow-harvested (BMH) CD34+ HSCs transduced with the BB305 LVV under original manufacturing. All patients had successful engraftment but levels of gene therapy-derived hemoglobin (HbAT87Q) were lower than expected. Despite this, hemolysis markers and the annualized rate of vaso-occlusive crisis (VOCs) plus acute chest syndrome (ACS) were reduced post-infusion. Here, we provide an update on these patients and explore factors that may contribute to the clinical benefit. Data on patients treated more recently are presented separately.
Methods
Adults with SCD-related complications (previously described) were enrolled in HGB-206. All patients received myeloablative busulfan conditioning before DP infusion. The initial group (Group A; N=7) was treated with DP from BMH HSCs using the original LentiGlobin manufacturing process. The protocol was amended, and 2 patients were subsequently treated in Group B with DP from BMH HSCs. Patient 1 had DP made with original and refined manufacturing processes and patient 2 had DP made only with the refined process. Patients were followed for 2 years in HGB-206 and offered participation in the LTF-303 study for long-term follow-up. Adverse events (AEs), Hb fractions, and additional laboratory and clinical parameters were monitored.
Results
As of 7 March 2019, the median follow-up post-DP infusion was 35.8 (min-max: 29.8-44.5) months in Group A; it was 17.2 and 20.2 months for Group B patients 1 and 2. There was full hematological recovery with no graft failure. The safety profile of LentiGlobin post-DP infusion was consistent with myeloablative busulfan conditioning and underlying SCD. No cases of Grade ≥ 3 DP-related AEs, veno-occlusive liver disease, vector-mediated replication competent lentivirus or clonal dominance were observed. Three years after LentiGlobin gene therapy, one Group A patient developed myelodysplastic syndrome, reported as unlikely related to LentiGlobin. The patient subsequently received an HLA-haploidentical donor transplant.
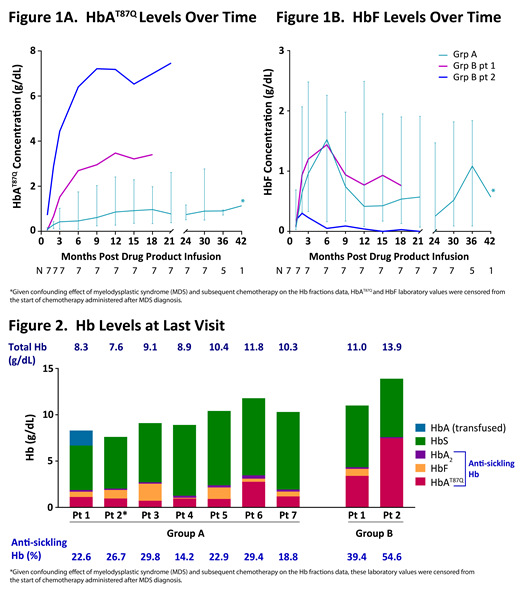
The annualized rate of VOCs plus ACS from LentiGlobin infusion to last follow-up was reduced by a median of 89 (min-max 10-100) % compared to that in the 2-year interval before enrollment in the 8 patients who had a history of VOC and/or ACS. One Group A patient who had a stroke pre-DP infusion has not had any red blood cell (RBC) transfusions through ~3 years post-DP infusion and no stroke recurrence. Median HbAT87Q levels were 1.0 (min-max 0.7-2.8) g/dL for Group A and 3.4 g/dL for Group B patient 1 at last visit and were stable for up to 3.5 years follow-up (Figure 1A). In several patients, HbF levels increased post-LentiGlobin treatment, peaking at ~2-6 months, and remaining higher at last visit compared to 1-month post-DP infusion. At last visit, the median HbF level was 0.6 (min-max 0.1-1.8) g/dL for all Group A and 0.8 g/dL for Group B patient 1 (Figures 1B and 2). In these patients, the median fraction of anti-sickling Hb (HbAT87Q + HbF + HbA2) was 22.9 (min-max 14.2-29.8) % and 39.4%, respectively. Patient 2 in Group B, who had DP made entirely using refined manufacturing process, had no HbF but produced high levels of HbAT87Q (7.5 g/dL) that contributed to 53.7% of total Hb.
Summary
In the HGB-206 Group A patients, the modest expression of gene therapy-derived HbAT87Q is accompanied by an induction of HbF. Elevated HbF levels have been shown to be associated with reduced severity of SCD. The resulting 14-30% of anti-sickling Hb observed in Group A patients, while not likely to be curative, showed clinical benefit as suggested by a reduction in the annualized rate of VOC plus ACS. Further, Group A and B patients have maintained HbAT87Q production, demonstrating the durability of gene therapy-derived β-globin gene expression. There have been no Grade ≥ 3 DP-related AEs in LentiGlobin-treated patients with up to 3.5 years of follow-up. Longer follow-up will help determine whether the initial induction of HbF, as is common after myeloablation, will be sustained and continue to contribute to therapeutic anti-sickling Hb levels.
Walters:Editas Medicine: Consultancy; TruCode: Consultancy; AllCells, Inc: Consultancy. Kwiatkowski:Apopharma: Research Funding; Imara: Consultancy; Celgene: Consultancy; bluebird bio, Inc.: Consultancy, Research Funding; Agios: Consultancy; Terumo: Research Funding; Novartis: Research Funding. Schmidt:German Cancer Research Center, Heidelberg, Germany: Employment; GeneWerk GmbH, Heidelberg, Gemrany: Equity Ownership. Miller:bluebird bio, Inc.: Employment, Equity Ownership. Pierciey:bluebird bio, Inc.: Employment, Equity Ownership. Huang:bluebird bio, Inc.: Employment, Equity Ownership. Ribeil:bluebird bio, Inc: Employment, Equity Ownership. Kanter:Rockpointe: Honoraria; GLG: Consultancy; Guidepoint Global: Consultancy; Novartis: Consultancy, Honoraria; Imara: Consultancy; Sangamo: Consultancy, Honoraria; Modus: Consultancy, Honoraria; Medscape: Honoraria; Peerview: Honoraria; bluebird bio, Inc.: Consultancy; SCDAA: Membership on an entity's Board of Directors or advisory committees; NHLBI: Membership on an entity's Board of Directors or advisory committees; Jeffries: Consultancy; Cowen: Consultancy. Thompson:bluebird bio, Inc.: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Baxalta: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal