Background
LentiGlobin for Sickle Cell Disease (SCD) gene therapy contains ex-vivo lentiviral vector (LVV)-mediated addition of a modified β-globin gene (βA-T87Q) into autologous CD34+ hematopoietic stem cells (HSCs). The safety and efficacy of LentiGlobin for SCD is being evaluated in the ongoing Phase 1/2 HGB-206 study (NCT02140554). Early data suggest that highly efficient HSC transduction and engraftment of long-term repopulating HSCs are needed to prevent sickle-related complications. Implementation of a refined manufacturing process resulted in a median vector copy number (VCN) of 3.8 (2.8-5.6) vector copies/diploid genome and median % transduced cells (% LVV+) of 80 (71-88) % in the drug product (DP). These DP improvements correlated with robust HbAT87Q production and better clinical outcomes in the recently treated cohort. Here we describe results of exploratory assays performed in this subset of patients to evaluate how DP characteristics impact SCD RBC physiology by assessing: 1) engraftment and persistence of LVV transduced cells in the bone marrow (BM) and peripheral blood (PB), 2) effect of VCN on βA-T87Q and βS levels in PBMC-derived erythroid colonies, 3) proportion of RBCs expressing βA-T87Q, and 4) impact of intracellular βA-T87Q and βS levels on RBC sickling. These assays are also underway in earlier study patients and will be presented.
Methods
Individual colonies were isolated from colony-forming unit (CFU) assays performed on DP (prior to infusion), CD34+ HSCs from BM aspirates post-DP infusion, and on PBMCs post-DP infusion. To evaluate engraftment of transduced cells, presence of LVV was determined by qPCR of individual colonies. βS expression in individual colonies was assessed by ultra-performance liquid chromatography. βA-T87Q expression in RBCs was assessed by a single cell western blot (scWB) assay, using concurrent staining with two antibodies, a novel antibody specific for βS and the other recognizing both βA and βA-T87Q. Percentage of sickled RBCs was quantified by imaging flow cytometry performed on RBCs exposed to 2% O2. Data are presented as median (min-max).
Results
The percentages of LVV+ colonies from PBMCs at 9 months and BM at 12 months post-infusion in 5 patients were 79.2 (67.0-88.4) % and 81.5 (60.6-88.1) %, respectively, consistent with stable engraftment of transduced cells. The proportion of erythroid and myeloid colonies from DP, BM and PBMCs with a given VCN was determined to assess the impact of VCN on engraftment of transduced cells. We observed a reduction in high-VCN progenitors obtained from PBMCs and BM post-infusion vs DP. PBMCs from one patient were used to generate erythroid colonies for VCN and HbS expression assessments: colonies with VCN = 1 had 62 (33-87) % contribution of βS and colonies with VCN ≥ 3 had 23 (0-55) % βS, suggesting a negative relationship between VCN and % βS expression.
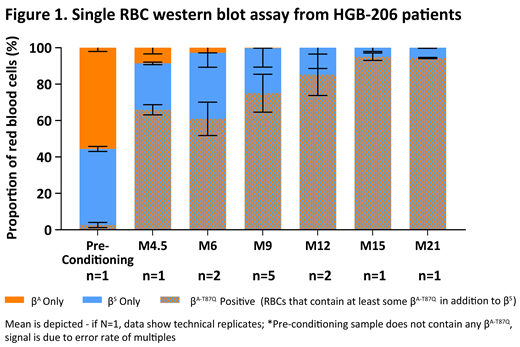
To investigate how the 80% LVV transduction efficiency in the DP relates to βA-T87Q expression in RBCs, we evaluated βS and βA-T87Q expression in individual RBCs by scWB assay at various time points after DP infusion (Fig 1). The proportion of RBCs positive for βA-T87Q at last study visit in 8 patients with ≥ 9 months of follow-up was 83.0 (65.5-95.8) %; with > 90% of RBCs positive for βA-T87Q in 4 patients.
The proportion of sickled RBCs was assessed from untreated patients with SCD, individuals with sickle cell trait and patients with ≥ 6 months of follow-up post-LentiGlobin treatment (n=7). The % sickled RBCs from LentiGlobin treated patients, while similar to that from trait individuals, was significantly lower compared to that in untreated patients with SCD.
Summary
These data demonstrate consistent engraftment and persistence of LVV-transduced cells following LentiGlobin gene therapy. The decrease in frequency of high-VCN progenitors from post-treatment PB or BM compared to DP suggests that these high-VCN progenitors may not contribute to gene-modified cell population in the long term. Further, these early data suggest that LentiGlobin gene therapy results in nearly pancellular βA-T87Q expression and reduction in βS expression,which impacts the pathophysiology of SCD as demonstrated by a reduction in RBC sickling. Together, these results begin to describe the complex relationship between characteristics of VCN and transduction efficiency in the HSCs in the DP, and the cellular physiology of erythroid lineage descendants after LentiGlobin gene therapy.
Bonner:bluebird bio, Inc.: Employment, Equity Ownership. Kanter:Novartis: Consultancy, Honoraria; Imara: Consultancy; Sangamo: Consultancy, Honoraria; Modus: Consultancy, Honoraria; Guidepoint Global: Consultancy; GLG: Consultancy; Cowen: Consultancy; Jeffries: Consultancy; Medscape: Honoraria; Rockpointe: Honoraria; Peerview: Honoraria; SCDAA: Membership on an entity's Board of Directors or advisory committees; NHLBI: Membership on an entity's Board of Directors or advisory committees; bluebird bio, Inc.: Consultancy. Macari:bluebird bio, Inc.: Employment, Equity Ownership. Lane:bluebird bio, Inc.: Employment, Equity Ownership. Lewis:bluebird bio, Inc.: Employment, Equity Ownership. Coles:bluebird bio, Inc.: Employment, Equity Ownership. Kassenaar:bluebird bio, Inc.: Employment, Equity Ownership. Mynampati:bluebird bio, Inc.: Employment, Equity Ownership. Schulze:bluebird bio, Inc.: Employment, Equity Ownership. Hebert:bluebird bio, Inc.: Employment, Equity Ownership. Walters:Editas Medicine: Consultancy; TruCode: Consultancy; AllCells, Inc: Consultancy. Thompson:Baxalta: Research Funding; Novartis: Consultancy, Research Funding; bluebird bio, Inc.: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Asmal:bluebird bio, Inc: Employment, Equity Ownership. Pierciey:bluebird bio, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal