Introduction: Philadelphia chromosome-positive acute myeloid leukemia (Ph+AML) is a rare form of AML and confers a dismal prognosis when treated with chemotherapy, with limited data available on the outcome when treated with allogeneic transplantation (allo-HCT).
Methods: Patients were selected from the Japanese nationwide transplantation registry database based on the following criteria: AML patients aged ≥16 years; first allo-HCT conducted between 1995 and 2016; complete remission at transplantation; and intermediate- or poor-risk cytogenetics. Patients with secondary AML or chronic myeloid leukemia in blast phase were not included. Ph+AML was defined as AML, including mixed phenotype acute leukemia (MPAL), that presented with t(9;22)(q34.1;q11.2) or BCR-ABL1 fusion transcripts at diagnosis. The primary endpoint was overall survival (OS) and secondary endpoints were relapse and non-relapse mortality (NRM). The probability of OS was estimated based on the Kaplan-Meier method and compared between the groups with log-rank test. The incidences of relapse and NRM were estimated using cumulative incidence analysis and compared between the groups with Gray's test. Multivariate analysis was performed using the Cox proportional hazards model. A matched-pair analysis was also performed based on the propensity score to minimize the confounding effect of baseline patient characteristics. Sex, age at HCT (<55 or ≥55 years), years of HCT (1995-2005 or 2006-2016), white blood cell count at diagnosis (≤20,000/µL or >20,000/µL), GVHD prophylaxis (cyclosporine- or tacrolimus-based), disease status at HCT (CR1/CR2 or CR>2), conditioning regimen (RIC or MAC), donor type (HLA-matched related/unrelated donor or alternative donor), MPAL or not, and performance status at HCT (0-1 or 2-4) were used to calculate the propensity score.
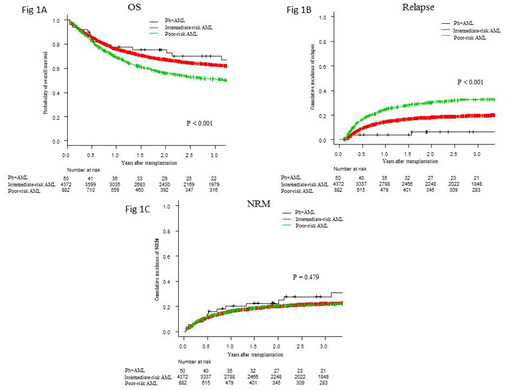
Results: Of 5,304 eligible patients, 4,372 (82.4%), 882 (16.6%), and 50 (1.0%) were classified into cytogenetically intermediate-risk AML, cytogenetically poor-risk AML (excluding Ph+AML), and Ph+AML groups, respectively. Their median age was 47, 47, and 43.5 (range: 16-76, 16-74, and 16-85) years, respectively. The median follow-up for surviving patients was 2.3 (range, 0.0-22.4) years. MPAL accounted for 1.7% of the whole cohort, and its distribution was different among groups: 1.3%, intermediate-risk AML; 2.4%, poor-risk AML; and 26.0%, Ph+AML (p < 0.001). No significant difference was noted in OS between patients with and without MPAL (p = 0.22). OS at 3 years after allo-HCT was 62.8% for intermediate-risk AML, 51.1% for poor-risk AML, and 70.2% for Ph+AML (p < 0.001) (Fig 1A). The 3-year incidences of relapse and NRM were 19.6% and 22.2% for intermediate-risk AML, 32.6% and 21.7% for poor-risk AML, and 6.3% and 27.9% for Ph+AML, respectively (Fig 1B and 1C). Multivariate analysis revealed that the Ph+AML and intermediate-risk AML groups had comparable risk in terms of OS [hazard ratio (HR), 0.75; 95% confidence interval (CI), 0.45-1.28; p = 0.29]; for these groups, the risk of Ph+AML was significantly lower than the poor-risk AML (HR, 0.54; 95% CI, 0.31-0.91; p < 0.001). Ph+AML had significantly lower risk of relapse than poor-risk AML (HR, 0.16; 95% CI, 0.05-0.51; p < 0.001) and intermediate-risk AML (HR, 0.31; 95% CI, 0.10-0.94; p = 0.04). There was no significant difference in the risk of NRM (HR, 1.45; 95% CI, 0.86-2.45; p = 0.17 for Ph+AML vs. intermediate-risk AML; HR, 1.50; 95% CI, 0.87-2.57; p = 0.14 for Ph+AML vs. poor-risk AML). The matched-pair analysis extracted 39 and 38 patients from each group based on the propensity score using optimum matching (intermediate-risk AML vs. Ph+AML and poor-risk AML vs. Ph+AML). OS in the Ph+AML group was comparable with that in the intermediate-risk AML group (p = 0.81), whereas it was significantly better than that in the poor-risk AML group (p = 0.012).
Conclusion: Our data show that post-transplant outcomes were better for Ph+AML patients than those with other poor-risk cytogenetics and were comparable with those for patients with intermediate-risk cytogenetics, suggesting that allo-HCT overcomes the poor prognosis of Ph+AML. Although our registry did not secure information on the use of tyrosine kinase inhibitors, their use may partly contribute to improvements in post-transplant outcomes.
Mizuno:Celgene Corporation: Honoraria; Bristol-Myers Squibb Corporation: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria. Kanda:Eisai: Consultancy, Honoraria, Research Funding; Taisho-Toyama: Research Funding; Tanabe Mitsubishi: Research Funding; Otsuka: Research Funding; Celgene: Consultancy, Research Funding; Dainippon Sumitomo: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Sanofi: Research Funding; Alexion: Consultancy, Honoraria; Mochida: Consultancy, Honoraria; Nippon-Shinyaku: Research Funding; Alexion: Consultancy, Honoraria; Chugai: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Mochida: Consultancy, Honoraria; Sanofi: Research Funding; Takara-bio: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; MSD: Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Nippon-Shinyaku: Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria, Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Tanabe Mitsubishi: Research Funding; Pfizer: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; CSL Behring: Research Funding; Pfizer: Research Funding; Dainippon Sumitomo: Consultancy, Honoraria, Research Funding; Asahi-Kasei: Research Funding; Novartis: Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Asahi-Kasei: Research Funding; CSL Behring: Research Funding; MSD: Research Funding; Taiho: Research Funding; Novartis: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Takara-bio: Consultancy, Honoraria; Taisho-Toyama: Research Funding; Taiho: Research Funding. Atsuta:CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal