Introduction
Aggressive natural killer cell leukemia (ANKL) is a rare leukemic form of mature natural killer cell neoplasms that is closely associated with Epstein-Barr virus. ANKL presents a fulminant clinical course, resulting in a poor prognosis with a median overall survival of approximately 2 months. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently the only curative treatment, but the long-term outcomes after allo-HSCT remain unclear.
Methods
Using the national Japanese transplant registry database, the outcomes of 59 ANKL patients who underwent first allo-HSCT between 1997 and 2016 were analyzed. Correlation between groups was examined by the c2 test, Mann-Whitney U test, and Kruskal-Wallis test. Patient survival data were analyzed using the Kaplan-Meier method and compared by the log-lank test. The cumulative incidence of relapse and non-relapse mortality were calculated considering competing risks.
Results
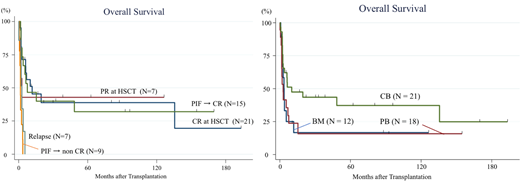
The median patient age was 37 years (range, 9 to 66), and males accounted for 68%. The median time from diagnosis to allo-HSCT was 3.7 months (range, 1.1 to 13.9). Twenty-nine patients received stem cells from cord blood (CB), 18 received them from peripheral blood (PB), and 12 received them from bone marrow (BM). Two patients had a prior history of autologous HSCT. Twenty-one patients (36%) had a complete response (CR) and 7 (12%) had a partial response (PR), but 31 (52%) were not responding at the time of allo-HSCT. Forty-four patients received myeloablative conditioning and 15 received non-myeloablative conditioning. Thirty-two patients received tacrolimus-based GVHD prophylaxis, including 1 with additional post-transplant cyclophosphamide as part of haploidentical HSCT, whereas 26 received cyclosporin-based GVHD prophylaxis. The median follow-up of survivors was 62 months (range, 0.9 to 193). The median OS and relapse-free survival were 3.9 months and 2.6 months, respectively. The probability of OS and relapse-free survival, and cumulative incidence of relapse and non-relapse mortality 1 year after HSCT were 33.9%, 32.4%, 55.5%, and 12.1%, respectively. The probability of OS was significantly higher for patients with CR or PR at allo-HSCT than for those without a response (40.6% vs 16.1% at 5 years; P = 0.046). Among the 24 patients with primary induction failure (PIF) at allo-HSCT, 15 achieved CR after allo-HSCT. The prognosis of these 15 patients was almost equivalent to that of those who received allo-HSCT in CR or PR, as shown in the Figure (P = 0.95). Regarding the stem cell source, the probability of OS was significantly higher for patients who received stem cells from CB than for those who received them from PB or BM (CB 37.3% vs PB 15.8% and BM 16.7% at 5 years; P = 0.04). Seventeen patients (59%) of those who received stem cells from CB were CR at HSCT, whereas only 4 patients (13%) of those who received stem cells from PB or BM were CR at HSCT. In addition, 5 (83%) of 6 patients who received stem CB transplantation in PIF achieved CR after allo-SCT, whereas 10 (56%) of 18 patients who received PB stem cell transplantation or BM transplantation in PIF achieved CR. The age and year at HSCT were not different between the groups. The time from diagnosis to allo-HSCT did not differ among stem cell sources (median CB 3.3 months, PB 3.7 months and BM 4.1 months; P = 0.31). Regarding the conditioning regimen, the probability of OS was not different between myeloablative and non-myeloablative conditioning regimens (P = 0.58). Patients who developed acute GVHD grade 1/2 had a significantly better prognosis than those with grade 3/4 or without GVHD (P < 0.001). In contrast, chronic GVHD development did not affect the prognosis (P = 0.60). At the last follow-up, 42 patients (71%) had died. The most common cause of death was primary disease (62%), followed by infection (14%) and organ dysfunction (7%).
Conclusion
Allo-HSCT can lead to long-term survival even for patients with PIF at HSCT. CB is useful as a stem cell source, providing good outcomes and timely allo-HSCT for this rapidly progressive disease. To confirm our findings and evaluate the outcome of allo-SCT in more detail, further studies including patients who did not receive allo-SCT for ANKL are warranted.
Ishida:Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding; Astellas Pharma: Research Funding; Eli Lily and Company: Research Funding; Celgene: Honoraria; Chugai Pharmaceutical: Consultancy, Research Funding. Izutsu:Celgene: Consultancy, Research Funding; Chugai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Astra Zeneca: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Symbio: Research Funding; Ono: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Solasia: Research Funding; Zenyaku: Research Funding; Incyte: Research Funding; Novartis: Honoraria, Research Funding; Sanofi: Research Funding; HUYA Bioscience: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Astellas Amgen: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; ARIAD: Research Funding; Takeda: Honoraria, Research Funding; Pfizer: Research Funding; Kyowa Kirin: Honoraria; Nihon Medi-physics: Honoraria; Janssen: Honoraria; Dainihon Sumitomo: Honoraria; Bristol-Byers Squibb: Honoraria; Mundi: Honoraria; Otsuka: Honoraria; Asahi Kasei: Honoraria. Suzumiya:Celgene, Kyowa Kirin, Chugai-Roche, Eisai, Takeda, Celltrion, SymBio, Astellas, Ono, AstraZeneca, Ootsuka, Taiho, Mundi, Dainihon-Sumitomo: Research Funding. Mitsui:MSD pharmaceutical: Research Funding; Maruho pharmaceutical: Research Funding; JCR pharmaceutical: Research Funding; Teijin pharmaceutical: Research Funding; Chugai pharmaceutical: Research Funding; Daiichi Sankyo pharmaceutical: Research Funding; Astellas pharmaceutica: Research Funding; Shionogi pharmaceutical: Research Funding. Kanda:Kyowa Hakko Kirin: Honoraria; Otsuka: Honoraria; Daiichi Sankyo Company: Honoraria; MSD: Honoraria; Chugai: Honoraria; Bristol-Meyers Squib: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Astellas: Honoraria; JCR Pharmaceuticals: Honoraria; Takeda: Honoraria; NextGeM Incorporation: Patents & Royalties: 2019-011392. Atsuta:CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria. Suzuki:Celgene: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; Kyowa Hakko Kirin: Honoraria; Chugai Pharmaceutical Co.,Ltd.: Honoraria; Meiji Seika: Honoraria; Bristol-Myers Squibb: Honoraria; Merck Sharp & Dohme: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; Eisai: Honoraria; ONO Pharmaceutical Co., Ltd.: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal