Background: Recipient immunity is compromised after HSCT, obligating patients (pts) to take prophylactic antimicrobial and antiviral agents and to be reimmunized to viral and bacterial pathogens. Hepatitis B virus (HBV) infection is a major public health problem, with about 30% of the world population having serological evidence of current or past HBV infection. HBV vaccination post-HSCT is imperative in these pts, as most lose protective HBV surface antibodies (anti-HBs) following conditioning, placing them at high risk for HBV reactivation. Guidelines recommend delaying vaccination (including HBV) for 6-12 months following transplantation to allow for cellular and humoral immune recovery. Even with delaying vaccination, immunosuppression, graft-versus-host disease (GVHD), and delayed immune reconstitution hinder the effectiveness of vaccines. The efficacy of HBV vaccination is not well defined in pts on immunosuppressive therapy (IST) and/or in those with GVHD. Further, little data exists on the efficacy of HBV revaccination in pts failing to respond to the 1st vaccination series. We studied factors impacting the success of vaccination in pts undergoing one to four HBV vaccination series after HSCT.
Methods: This single-center, retrospective study evaluated the effectiveness of HBV vaccine in HSCT pts by assessing protective antibody generation after vaccination. Fifty-two pts (25 female, 27 male) who received at least one 3-dose HBV vaccination series post-HSCT and who had evaluable post-vaccine anti-HBs titers were included in the analysis. Pts with negative or indeterminate anti-HBs titers following the first vaccine series were eligible to receive one or more additional series of HBV vaccinations. All pts were treated with cyclophosphamide and fludarabine based conditioning (± anti-thymocyte globulin) and received GVHD prophylaxis with either cyclosporine/tacrolimus with or without mycophenolate mofetil. The vaccine response rate over a series of vaccinations was estimated by Kaplan-Meier methods. The development of response after the first vaccination was correlated with patient baseline and post-HSCT factors including pretransplant HBV titers, vaccination time post-transplant, use of rituximab and IST and absolute lymphocyte count (ALC), CD4, and CD8 cell counts and history of acute or chronic GVHD.
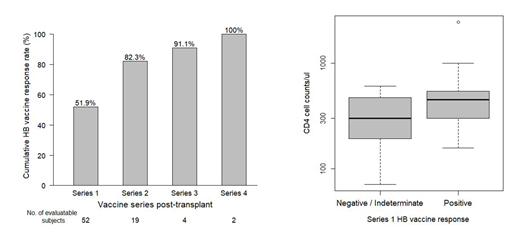
Results: The studied cohort included 52 HSCT pts with a median age of 22 years (range 7-62) and a variety of diagnoses (38 aplastic anemia, 6 myelodysplastic syndrome and 8 hematological malignancies). Thirty-five pts underwent HSCT from an HLA-matched donor and 17 pts received a combined haploidentical and umbilical cord blood transplant. The median time to first HBV vaccination was 12 months (8-37) post-HSCT. Following the 1st vaccination series; 19, 4 and 2 pts received a 2nd, 3rd and 4th vaccination series. The estimated cumulative anti-HBs response rates were 51.9%, 82.3%, 91.1% and 100% for the four vaccination series, respectively (Figure). A logistic regression analysis revealed: a) Pts who achieved a response after the initial vaccination series had higher CD4 counts compared to those who failed to mount a response (median CD4 count 450 vs. 300/μL, P= 0.024, Figure); b) Pts without a history of acute GVHD (n=23) were significantly more likely to respond to the 1st vaccination series compared to those with acute GVHD (n=29) (response: 69.6% vs 37.9%, P= 0.029). Other factors included in this analysis were not found to be correlated with the anti-HBs response after the initial vaccination series.
Conclusions: Multiple rounds of HBV vaccination may be required before a protective antibody response is achieved. After the first vaccination series, only 51.9% of pts achieved a response, with lower pre-vaccination CD4 counts and a prior history of acute GVHD being negatively associated with vaccine success. Remarkably, with continued vaccination attempts (up to four vaccination series), all evaluable pts ultimately developed a protective anti-HBs response.
Shalabi:GlaxoSmithKline: Other: Spouse is employed by GSK Pharma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal