Background: Allogeneic stem cell transplantation (AlloSCT) from an HLA-matched sibling donor is the only known curative therapy in patients with high-risk SCD (Talano/Cairo, EJH, 2015). Unfortunately only about 15% of high risk patients with SCD have an HLA-matched unaffected sibling donor. T cell depletion has been employed to reduce AGVHD e.g., CD3/CD19 cell depletion (Barfiled RC, et al, Cytotherapy, 2004), αβ T-cell/CD19 cell depletion (Locatelli F, et al, Blood, 2017), CD34+ positive selection (Aversa F, et al, NEJM, 1998). MUD transplantation in high-risk SCD recipients has shown unexpectedly high rates of CGVHD (Shenoy et al, Blood, 2016). We reported a very low incidence of acute and chronic GVHD in pediatric recipients receiving CD34 enriched HPC products with PB MNC addback with 2 x 105 CD3/kg from MUD donors (Geyer/Cairo et al, BJH, 2012). Furthermore, rapid NK cell reconstitution after AlloSCT is associated with a significant improvement in 1yr OS (Pical-Izard, BBMT, 2015; Dunbar et al, Hematologica, 2008). Recently, we reported promising results for high-risk SCD patients at 1 year follow-up after FHI CD34 enriched/PBMNC with addback AlloSCT with the probability of 1-year overall survival (OS) n=17; 88.2% (CI95: 60.6-96.9) (Talano/Cairo, ASH, 2017), expanding the donor pool and hopefully improving outcomes for high-risk patients with SCD.
Objective: To investigate donor chimerism, immune cell reconstitution and NK cell function in high-risk patients with SCD following AlloSCT using FHI CD34 enrichment/PBMNC (2 x 105 CD3/kg) addback.
Methods: Twenty-one eligible SCD patients (2-<21 yrs) were enrolled. Nineteen patients received hydroxyurea, azathioprine, fludarabine, busulfan, thiotepa, cyclophosphamide, R-ATG, and TLI followed by FHI AlloSCT to date (Talano/Cairo, ASH, 2017). CD34 cells were enriched using the CliniMACS® system, kindly provided by Miltenyi Biotec, with a target dose of 10 x 106 CD34+ cells/kg with a PBMNC addback dose of 2x10*5 CD3/kg in the final product. Whole blood and RBC chimerism (estimated using CD71 to isolate an eythroid lineage-enriched fraction) were determined by STR. Immune cell and subset reconstitution was assessed by flow cytometry as previously described (Geyer/Cairo et al. BJH, 2012). NK function was determined by cytotoxic activity against K562 tumor targets at 10:1 E:T ratio by europium release assay and intracellular LAMP-1 (CD107a) and granzyme B expression by flow cytometry as previously described (Chu/Cairo et al, Can Imm Res, 2015).
Results:
There was 100% engraftment of neutrophils and platelets. The median day post-HISCT to neutrophil and platelet engraftment was +9 and +19, respectively.
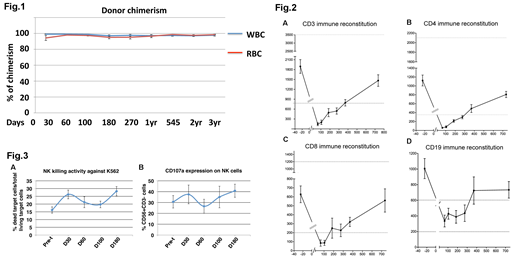
Whole blood donor chimerism (mean±SEM) at 1-year, 2-year, and 3-year post-HISCT was 97±1%, 97±1%, 97±1%, respectively (Fig.1). Donor chimerism for CD71+ RBCs (mean±SEM) at 1-year, 2-year, 3-year post-HISCT was 97±2%, 98±1%, 98±1%, respectively (Fig.1).
Immune reconstitution of CD3, CD4, CD8, and CD19 was evaluated. The time to recovery of minimally normal levels post-HISCT of CD3 (800 cells/ul), CD4 (400 cells/ul), CD8 (200 cells/ul), and CD19 (200 cells/ul), was approximately 365, 365, 270, and 60 days post-HISCT (Fig.2), respectively. Probability of Grade II-IV AGVHD, CGVHD and 1 year EFS/OS was 6.2%, 6.7% and 90%, respectively.
NK reconstitution was rapid and peaked at d+30 (36±9%, 2710cells/ml). NK cytotoxicity against K562 at a E:T=10:1 peaked at d+30 (26±3%) and d+180 (28±3%) vs at pre-t (16±2%) (p<0.01) (Fig. 3A). Consistent with increased NK cytotoxicity, CD56dimCD3- subset was increased at d+30 vs pre- HISCT (p<0.05). The NK activation marker, CD107a peaked at d+30 (38±9%) and d+180 (41±6%) (Fig.3B). More over, reconstituted NK cells expressed higher level of activating receptors NKp46 (24±9%), NKG2D (32±9%) and KIR2DS (8±3%) and inhibitory receptors NKG2A (33±9%), CD94 (28±9%) and KIR2DL2/3 (11±2%) at d+30 compared to other time points.
Conclusion:
Despite a 5 log depletion of T cells, the PBMNC addback (fixed at 2 x 105 CD3/kg) facilitated rapid donor chimerism and immune reconstitution with a low probability of Grade II-IV AGVHD. The rapid NK reconstitution may have in part contributed to the excellent 1yr OS in the FHI study. (Supported by FDA R01FD004090 (MSC)).
Cairo:Jazz Pharmaceuticals: Other: Advisory Board, Research Funding, Speakers Bureau; Osuka: Research Funding; Miltenyi: Other: MTA.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal