Background: Endothelial Activation and Stress Index (EASIX) was developed as a simple surrogate of endothelial dysfunction and when evaluated pre-allogeneic hematopoietic cell transplantation (allo-HCT), is one of the strongest tools for predicting non-relapse mortality (NRM). In several datasets, we have found that high EASIX scores at days +30 and +100 post allo-HCT and at the onset of graft-versus-host disease (GVHD) are associated with higher NRM and poorer overall survival (OS) (data unpublished). These data demonstrate that EASIX analyzed as a categorical variable at specific landmarks is associated with outcomes after allo-HCT. However, the trend of EASIX scores has never been evaluated as a continuous variable over time. We hypothesized that defining the natural history of changes in EASIX post-HCT would help us identify an optimal time point at which EASIX has the highest discrimination for NRM. We also sought to determine whether changes in EASIX over time may be a more informative marker of NRM.
Methods: We evaluated 509 adult patients who received an unmodified or ex-vivo CD34+-selected allo-HCT between April 2008 and December 2016. One hundred and forty-nine patients underwent unmodified, reduced intensity or nonmyeloablative allo-HCT with uniform GVHD prophylaxis of sirolimus/tacrolimus and low-dose methotrexate. Three hundred and sixty patients underwent myeloablative allo-HCT with ex-vivo CD34+ selection (CliniMACS® CD34 Reagent System) as GVHD prophylaxis. The EASIX score (LDH*creatinine/platelet count) was calculated at continuous timepoints from baseline [day -30 to day -10] until 1-year post-HCT. For each longitudinal evaluation, the concordance of EASIX was estimated for NRM events occurring in the subsequent 180 days. A log transformation using base 2 (log2) was applied to all EASIX variables to reduce skew. A one-unit increase in log2 EASIX is associated with a doubling (one-fold increase) of EASIX on the original scale. Disease relapse or death were considered competing risks for NRM.
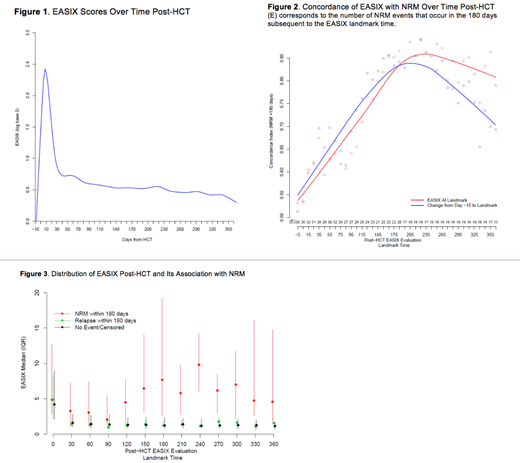
Results: Patient and HCT characteristics are detailed in Table 1. Median age at HCT was 56 years (range 19-78) and 59% of patients were males. The majority of unmodified allo-HCT were done for non-Hodgkin lymphoma (69%), while the majority of CD34-selected allo-HCTs were done for acute leukemia (61%). Most patients had sensitive disease at time of HCT (CR=61%; PR=13%). All patients except two received peripheral blood mobilized allografts. HCT-CI was 0 in 24% of patients, 1-2 in 32% and ≥ 3 in 44%. Sixty-eight patients experienced NRM within 1-year post-HCT. Causes of death in these patients were infection (41%), GVHD (29%), toxicity/organ failure (24%) and other (6%). Among all patients, EASIX scores rise rapidly early post-HCT and peak day +8 followed by sharp decline until day +40. Thereafter, EASIX scores tend to downtrend, but remain above baseline for the duration of the first year post-HCT (Figure 1). EASIX discrimination of 180-day NRM increases from time of allo-HCT until day +180 to +210, when concordance is highest (concordance index=0.85) (Figure 2). Overall, the ability of the EASIX score to discriminate NRM event times is similar when EASIX is analyzed as a categorical variable at landmark timepoints and as change from pre-HCT baseline EASIX score. In the first days post-HCT, EASIX values rise to a similar degree in patients regardless of whether they experience NRM or relapse in the following 180 days. Later in the post allo-HCT course, patients who do not experience NRM, including patients who relapse, have consistently lower EASIX scores compared to those who experience NRM in the following 180 days (Figure 3).
Conclusions: Our data are the first to characterize the continuous trend of EASIX scores after allo-HCT, demonstrating that EASIX scores are highly dynamic and have variable concordance with NRM when analyzed longitudinally. While pre-HCT EASIX can be used to help guide allo-HCT treatment decisions prior to allo-HCT, evaluation of the dynamic changes in EASIX scores may better predict risk of NRM over time as patients acquire additional endothelial injury and toxicities after HCT. Assessment of dynamic EASIX scores may be useful in guiding novel investigative approaches to reduce the risk of toxicities and NRM along the allo-HCT journey.
Giralt:Actinium: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy; Takeda: Consultancy, Research Funding; Kite: Consultancy; Miltenyi: Research Funding. Perales:Miltenyi: Research Funding; Kyte/Gilead: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Medigene: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Scordo:McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal