Introduction
Nearly all adoptive cell therapies currently being evaluated in the clinic, including CAR-T, TIL, and TCR-based cell therapies, require lymphodepletion to remove cellular cytokine sinks and create a favorable cytokine environment for the incoming transferred cells to proliferate. Targeted conditioning with an antibody radio-conjugate directed to CD45 represents a promising and potentially more effective alternative to the commonly used fludarabine/cyclophosphamide chemotherapy lymphodepletion regimen.
Methods
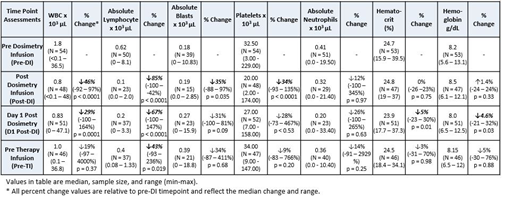
SIERRA is an ongoing Phase 3 multicenter trial evaluating anti-CD45 Iodine (131I) Apamistamab [Iomab-B] as targeted conditioning prior to HCT in active, relapsed or refractory acute myeloid leukemia. Prior to administration of the therapeutic dosage, dosimetry is performed using a tracer amount of Iomab-B (range from 7-20 mCi, median 10 mCi) in an out-patient setting to calculate the appropriate patient-specific therapeutic infusion. Blood sample analysis from 57 evaluable Iomab-B treated patients collected pre-dosimetric infusion (Pre-DI), post-dosimetric infusion (Post-DI), day 1 post-dosimetric infusion (D1 post-DI), and pre-therapeutic infusion (Pre-TI, range 6-14 days post-dosimetry) was assessed to determine if residual Iomab-B had any significant effect on blood counts in support of its use as a transient targeted lymphodepletion agent.
Results
From these data, a significant but transient decrease in lymphocytes and white blood cells was observed compared to pre-DI values. An 85% decrease of lymphocytes was observed at the post-DI time point, a 67% decrease at day 1 post-DI, and a 43% decrease at the time of therapeutic infusion. Peripheral blasts were also transiently decreased at the post-DI time point (35%), indicating that low dose Iomab-B may exert an anti-tumor effect in these patients. Interestingly, the levels of platelets, hematocrit, and neutrophils were unchanged at the Pre-TI time point compared to Pre-DI, reflecting the comparatively lower surface antigen levels of CD45 on these cell types. In addition, data from a subset of treated patients (n=25) was used to calculate the radiation absorbed dose to bone marrow to determine an appropriate amount of Iomab-B that would not impart more than 2 Gy, a threshold that is considered to be non-myeloablative. This analysis determined that 75 mCi Iomab-B could be administered as a non-myeloablative amount and has been proposed as the starting dose for a clinical trial using Iomab-B for targeted lymphodepletion prior to CAR-T. Additional calculations were performed to model the clearance of Iomab-B to determine at what time post-infusion a CAR-T could be administered without the amount of residual radiation to bone marrow exceeding a safe level (0.25 Gy). Based on clinical data from the SIERRA trial, the average effective half-time of Iomab-B was 45.1 hours and the time frame for CAR-T administration following 75 mCi of Iomab-B was 136 hours (5.7 days). Given that administration of radiopharmaceuticals often requires special safety precautions, the proposed range of doses for Iomab-B is considered an outpatient infusion without the need for isolation.
Conclusions
Despite the importance of lymphodepletion prior to adoptive cell therapies, there has been very little optimization of this step. Clinical data collected using a low dose of Iomab-B for dosimetry has demonstrated that this method of lymphodepletion is specifically targeted to CD45+ immune cells, may have an anti-tumor effect, and can be administered in an outpatient setting. These clinical and logistical attributes are attractive characteristics for lymphodepletion and supportive of using Iomab-B as a novel lymphodepletion regimen prior to adoptive cell therapies such as CAR-T.
Nath:Astellas: Consultancy; Daiichi Sankyo: Consultancy; Actinium: Consultancy. Geoghegan:Actinium Pharmaceuticals: Employment. Spross:Actinium Pharmaceuticals: Employment, Equity Ownership. Lichtenstein:Actinium Pharmaceuticals: Employment, Equity Ownership. Konerth:Versant Medical Physics and Radiation Safety: Consultancy. Fisher:Versant Medical Physics and Radiation Safety: Employment. Liang:Actinium Pharmaceuticals: Employment. Ludwig:Actinium Pharmaceuticals: Employment, Equity Ownership. Reddy:Actinium Pharmaceuticals: Employment. Berger:Actinium Pharmaceuticals, Inc: Employment, Equity Ownership. Gyurkocza:Actinium Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal