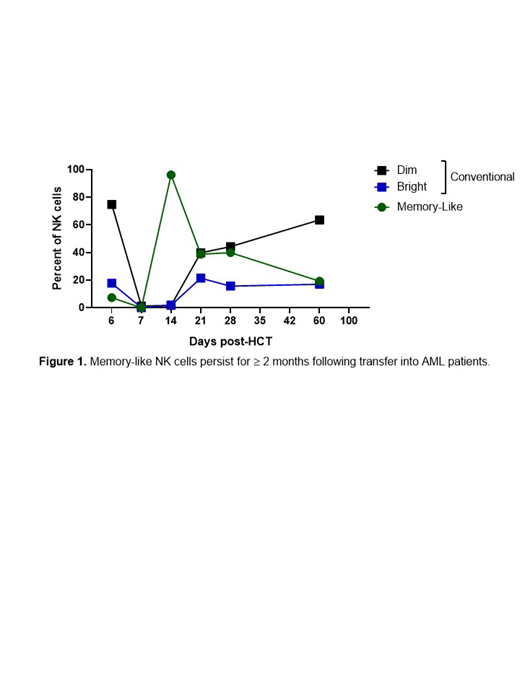
Natural killer (NK) cells exhibit innate memory or memory-like responses following stimulation with haptens, viruses, or cytokines. Human memory-like (ML) NK cells differentiate following a short-term activation with IL-12, IL-15, and IL-18, and have increased anti-tumor activity against AML and other cancers in vitro, in xenograft models, and in the first-in human phase 1 clinical trial of ML NK cells in AML (PMID27655849). In this trial, CR/Cri was observed in >50% of patients treated, and mass cytometry revealed a unique multi-dimensional phenotype of in vivo differentiated ML NK cells that was confirmed using donor-specific HLA markers. Although adoptively transferred MHC-haploidentical ML NK cells expanded and differentiated over 2-3 weeks, these cells were eliminated by recipient allogeneic immune responses, a challenge observed with all allogeneic lymphocyte therapies. The immune rejection observed in the allogeneic setting precluded following ML NK phenotype, persistence, and function long-term in these patients. We hypothesized that ML NK cells could persist longer than 2-3 weeks in an MHC-compatible setting, and thus be able to assess ML NK cells durability. To test this idea, a phase 2 clinical trial was designed for relapsed/refractory AML patients, who receive a reduced-intensity HLA-haploidentical hematopoietic cell transplant (HCT), followed by same-donor ML NK cell adoptive transfer at day 7, with 2 weeks of IL-15/N-803 support (NCT02782546). Using mass cytometry, ML NK cells were confirmed as distinct from conventional NK cells, CD56hi/NKp30hi/CD62Lhi/KIR+/NKG2A+/CD57+/-, by viSNE analysis, and clearly inconsistent with immature NK cells arising from the HCT graft (CD56bright/KIR-/CD57-). ML NK cells persisted in patients for at least 2 months (n=5) following adoptive transfer, and constituted 20-50% of total NK cells at day 60 (n=3, 206±97 cells/μl; mean±SEM; peak ML NK cells = 751-1106 cells/µl, D21-D28, n=5). These ML NK cells appeared highly functional (56±8% IFN-γ+, 20±3% TNF+, 41±7% CD107a+) when stimulated with tumor targets immediately ex vivo on study day 28 (n=7). Unsupervised clustering of scRNA-seq from patient samples acquired 14-60 days after ML NK cell adoptive transfer identified a subset of NK cells transcriptionally distinct from conventional CD56bright and CD56dim. This NK cell population was the majority of NK cells at study Day 21 and remained identifiable 2 months post-transfer. In agreement with the mass cytometry data, these NK cells expressed high levels of KIRs with scRNA-seq analysis uncovering novel transcriptional changes in granzyme M, perforin, KLRG1, and IFNG suggesting ML NK cells represent a mature, activated NK cell subset distinct from conventional NK cells arising from the graft. In addition, scRNAseq analysis identified high expression of the transcription factor RUNX3, a potential regulator of ML NK cell phenotype in vivo. In conclusion, a single infusion of ML NK cells resulted in a durable population of highly functional NK cells, as evidenced by multi-dimensional analyses using mass cytometry and scRNA-sequencing. These studies provide evidence that ML NK cell therapy in the MHC-compatible setting overcomes persistence barriers and provide a platform for innovation in NK cell therapeutics.
Cashen:Celgene: Other: Speaker's Bureau; Seattle Genetics: Other: Speaker's Bureau; Novartis: Other: Speaker's Bureau. Fehniger:Cyto-Sen Therapeutics: Consultancy; Horizon Pharma PLC: Other: Consultancy (Spouse).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal