Introduction: Donor plasmacytoid dendritic cells (pDCs) have important roles in the pathogenesis of graft versus host disease (GvHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Vasoactive intestinal peptide (VIP), a potent anti-inflammatory neuropeptide with pleiotropic effects, has shown promise in managing GvHD through induction of regulatory dendritic cells (Chorny, Blood 2006) and regulatory T cells (Delgado, JLB 2005). Our previous data showed that transplantation of hematopoietic stem cells (HSC) lacking VIP expression in enhanced Th1 polarization and antiviral immunity (Li, JI 2011). Whether VIP produced by pDCs plays a key role in preventing GvHD is the focus of the current study.
Method: A reductive allo-HSCT model was established by transplanting purified populations of 5 x 103 hematopoietic stem cells (HSC), 5 x 104 pDCs and 106 T cells from B6 (H-2Kb) to MHC mismatched B10.BR (H-2Kk) recipients. Mice received either VIP knockout pDCs (experimental group) or wild-type pDCs (control). Survival and clinical GvHD scores were monitored for at least 60 days. Serum pro-inflammatory cytokines were measured by luminex on day 3 and day 8. Additional recipients were transplanted for interim analyses of GvHD pathology in histological sections of small intestine and colon on day 8 and day 15. To investigate pDC migration and proliferation status, 5 x 104 luciferase-expressing pDCs, together with 5 x 103 WT B6 HSC, were transplanted to MHC mismatched Balb/c (H2kd) recipients. Bioluminescent imaging was performed on day 14.
We used mice carrying an eGFP reporter linked to a VIP promoter to measure VIP production by donor cells in vivo. The construct has the eGFP reporter gene, followed by a polyadenylation sequence, inserted into BAC clone RP23-25A8 at the initiating ATG codon of the first coding exon of the VIP gene, so that eGFP expression is driven by the regulatory sequences of the BAC gene. The BAC is inserted randomly in the genome. We used serial breeding to establish the eGFP-VIP reporter in B6 mice. Bone marrow pDCs were sorted from transgenic eGFP-VIP B6 mice and used in transplant experiments, allowing VIP gene activity from donor-derived pDCs to be detected as GFP expression using confocal microscopy.
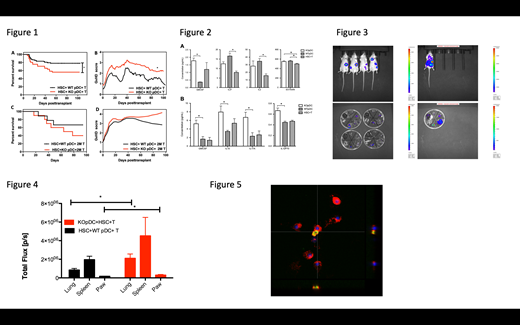
Results: Compared to recipients of WT pDCs, recipients of VIP-KO pDCs have a significantly lower survival rate with increased GvHD clinical score (Figure 1; *p<0.05). Transplant recipients of VIP-KO donor pDC had higher serum pro-inflammatory cytokine levels on day 3 and day 8 respectively. (Figure 2). At 2 weeks post-transplant donor pDCs migrated primarily to the spleen, with limited presence of donor pDCs in the gastrointestinal tract and lung (Figure 3). Compared to mice transplanted with WT pDCs, recipients of VIP-KO pDCs had increased donor T cell migration to the lung and paw, which are chronic GvHD target organs after two weeks. (Figure 4; *p<0.05). When stimulated with IFN-g, eGFP-VIP transgenic pDCs have activation of the VIP gene promoter in vitro, as detected using eGFP (Figure 5). Confocal microscopy data showed that pDCs have activated the VIP promoter and secreted more VIP following stimulation by IFN-g, consistent with VIP up-regulation by the inflammatory mileu in allo-transplant recipients.
Conclusions: These data show that lack of VIP secretion by donor pDCs resulted in lower survival rates and higher GvHD scores in allo-BMT recipients. Recipients of VIP KO donor pDC had increased donor T cell proliferation in GvHD target organs and increased GvHD clinical signs. The major site of donor pDCs migration in recipients is spleen, suggesting that pDCs may interact with other lymphoid cells, such as T cells, inducing a more tolerogenic environment. Donor pDCs were also found, to a lesser extent, in the lung and gastrointestinal tract, which are both GvHD target organs. Thus, pDCs may protect recipients from GvHD by limiting the activity of alloreactive T cells at these sites. The mechanism by which VIP-secreting donor pDCs regulate GvHD will be discussed.
Waller:Amgen: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Other: Travel expenses, Research Funding; Cerus Corporation: Other: Stock, Patents & Royalties; Chimerix: Other: Stock; Cambium Oncology: Patents & Royalties: Patents, royalties or other intellectual property ; Kalytera: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal