Background:
Hematopoietic stem cell (HSC) transplant is an essential treatment for a variety of blood disorders and malignancies. A key step in this procedure is the mobilization of donor stem cells. The most commonly used regimen for donor mobilization is a 5-day course of G-CSF. The length of this regimen coupled with the associated side effects emphasizes a need for superior alternatives. In recent years, there has been a growing understanding of mechanisms governing stem cell retention within the bone marrow niche. This has led to the development of new mobilization drugs that specifically target these processes. Two examples of previously described drugs that target mechanisms of stem cell retention are Plerixafor (a CXCR4 inhibitor already in clinical use), and truncated Gro-Beta (tGroβ; a CXCR2 agonist). Another potential target for inducing mobilization is disruption of the interaction between the VLA-4 integrin and its ligand VCAM-1. In this study, we evaluate the efficacy of novel VLA-4 inhibitors (VLA4i) alone and in combination with Plerixafor and/or tGroβ for the purposes of hematopoietic stem cell mobilization.
Methods:
We synthesized over 15 novel VLA-4 inhibitor molecules and tested their potency using soluble VCAM-1 binding assays. The 5 inhibitors determined to be most potent were then tested in vivo in DBA mice for their ability to mobilize HSCs alone and in combination with tGroβ and/or Plerixafor (n=5). HSC mobilization was measured in wild-type and splenectomized mice via flow cytometry to quantify the proportion of LSK (Lineage- Sca+ cKit+) cells as well as via Colony Forming Unit (CFU) assays. For competitive transplant, mobilized CD45.1+ BALB/c mouse blood (10 uL) was injected into lethally irradiated CD45.2+ BALB/c recipients alongside 2.5x105 CD45.2+ BALB/c bone marrow cells (n=10 / cohort). HSC engraftment was monitored monthly via flow cytometry for ratio of 45.1+ vs. 45.2+ cells in peripheral blood.
Results:
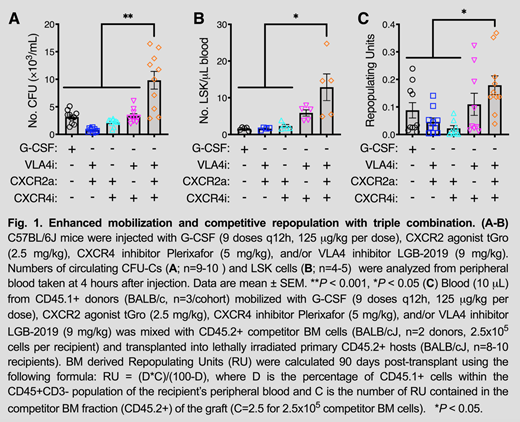
Firetagrast and BIO5192 are previously characterized VLA4i that have been administered to humans for indications unrelated to HSC mobilization. Our best VLA4i to date, LGB-2019, exhibited similar potency as BIO5192 in preventing the binding of sVCAM-1 to VLA-4 (IC50: 1.7nM) and was >200-fold more potent than firategrast. LGB-2019 showed increased aqueous solubility and mobilized 1.5-fold more murine LSK cells for a longer time period (peak HSC mobilization maintained for 4 hours) than BIO5192 when administered alone. Simultaneous injection of C57BL/6 mice with LGB-2019 (VLA4i), Plerixafor (CXCR4i) and tGro-β (CXCR2a) resulted in a synergistic increase in circulating CFUs (Fig. 1A; 9.8 x 103 CFUs/mL) and LSKs (Fig. 1B; 12.8 LSKs/uL) at 4 hours post-injection. In contrast, 5 days of G-CSF treatment mobilized approximately 3-fold and 8-fold less CFUs and LSKs, respectively (Fig. 1A-B). We saw no significant difference in mobilization for splenectomized vs. wildtype mice (23.4 x 103 CFUs/mL vs. 23.0 x 103 CFUs/mL) when mobilizing DBA/2 mice via VLA4i+CXCR4i+CXCR2a. Three months after competitive transplantation, blood obtained from BALB/c mice mobilized with the triple combination engrafted significantly better than blood obtained from mice treated with G-CSF or the dual combinations (Fig. 1C).
Summary:
New insights about the stem cell niche have allowed for the development of targeted drugs for the purposes of mobilization. Here, we show that a novel VLA-4 receptor inhibitor in combination with two other known mobilizers induces mobilization of hematopoietic stem and progenitor cells (CFU/LSK) at levels superior to the standard of care G-CSF and in a dramatically shortened time frame. Mouse transplant data also show superior engraftment in lethally irradiated recipients when using the triple cocktail regimen compared to the G-CSF mobilized graft. Secondary transplants are ongoing and will provide a more complete picture of primitive HSC mobilization and serial engraftment properties of the cells.
Rettig:WashU: Patents & Royalties: Patent Application 16/401,950. Karpova:WashU: Patents & Royalties: Patent Application 16/401,950. Ruminski:WahU: Patents & Royalties: Patent Application 16/401,950. Morrow:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. DiPersio:Cellworks Group, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners Arch Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Equity Ownership; Incyte: Consultancy, Research Funding; Bioline Rx: Research Funding, Speakers Bureau; Macrogenics: Research Funding, Speakers Bureau; Karyopharm Therapeutics: Consultancy; Celgene: Consultancy; Amphivena Therapeutics: Consultancy, Research Funding; WUGEN: Equity Ownership, Patents & Royalties, Research Funding; NeoImmune Tech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal