Background: Lenalidomide maintenance therapy post-autologous stem cell transplantation (ASCT) is associated with improved progression-free survival (PFS) and possibly overall survival in multiple myeloma (MM). However, almost all patients do relapse as a result of residual multiple myeloma cells that remain after the high-dose chemotherapy. In the myeloma setting it has been found that the hedgehog (Hh) pathway is essential for maintaining a subset of tumor causing stem cells. LDE 225 (Sonidegib) is a potent selective oral bioavailable antagonist of Smoothened (SMO), a component of the Hh signaling pathway. In in vitro experiments, LDE225 treatment of myeloma cell lines resulted in a modest inhibition of cell proliferation at increasing doses. When LDE225 was combined with lenalidomide, a more than additive effect was observed in terms of cell proliferation, an effect that was more pronounced in the context of myeloma cell lines growing in co-culture with marrow derived stromal cells. These findings form the basis of evaluation of LDE 225 as a strategy to enhance the activity of lenalidomide in the post-transplant maintenance setting. The minimal residual state post SCT provides the most optimal situation for evaluation of a drug that is likely to work by inhibiting the tumor cells that escaped high dose therapy.
Methods: Multiple myeloma patients without evidence of progression, who were 60 - 120 days after a single autologous stem cell transplant (SCT), performed within 1 year of diagnosis were eligible for the study. Maintenance therapy was started approximately 3 months after SCT. Treatment consisted of lenalidomide 10 mg days 1-21 and LDE225 400 mg days 1-28 in 28-day cycles for a total of 18 cycles. The goal of the study was to assess toxicity of this combination, complete response rate (CR) progression free survival (PFS) at 1 and 2 year and overall survival (OS). CR and PFS were estimated using an exact binomial distribution and Kaplan Meier curves respectively.
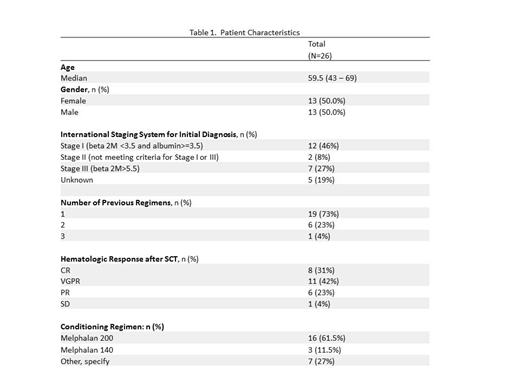
Results: A total of 28 patients were accrued from Jan 2014 to Aug 2016, 1 patient canceled prior to treatment and 1 patient was deemed ineligible resulting in 26 evaluable pts for CR and PFS. The median age of all pts (n=26) was 60 years (range 43-69) and 50% were males. Seventy-three percent of patients reported one treatment regimen prior to SCT, while 27% reported 2 or more prior regimens. The other characteristics of the patient are summarized in Table 1. Twenty seven pts received at least one cycle of treatment and are evaluable for toxicities (AE). Patients were treated for a median of 12.5 (range 1-18) cycles. While 10 pts (38.5%) completed protocol treatment (18 cycles), the remaining 16 pts went off treatment due to AEs (6, 23%), disease progression (3, 11.5%), refusal of further treatment (3, 11.5%) and other reasons (4, 15.4%). A grade 3 or higher AE at least possibly attributed to either drug was seen in 63%. Grade 3+ hematologic toxicities were noted in 30%, with 7% neutropenia and 4% thrombocytopenia. Notable grade 2+ non-hematologic toxicities with more than 5% incidence were dysgeusia 22%, alopecia 11%, and anorexia 7%. Grade 3+ non-hematologic toxicities were fatigue, myalgia and arthralgia each at 7%. The CR rate in evaluable patients was 46% (5 CRs and 7 sCRs) with a 95% CI of 27% - 66%. CR rate improved from 31% to 46%. VGPR or better improved from 42% to 85%. The 24-month PFS (time from SCT to progression or death due to any cause) was 73% (95% CI: 57.9 - 92.3%) with a median time to censoring of 38 months.
Conclusion: Lenalidomide in combination with LDE225 as posttransplant maintenance therapy was associated with some toxicity but manageable. The combination improved the depth of response after autologous stem cell transplant. Long-term follow-up is needed to determine overall survival.
Lacy:Celgene: Research Funding. Dispenzieri:Celgene: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Janssen: Consultancy; Intellia: Consultancy; Akcea: Consultancy; Alnylam: Research Funding. Gertz:Ionis: Honoraria; Alnylam: Honoraria; Prothena: Honoraria; Celgene: Honoraria; Spectrum: Honoraria, Research Funding; Janssen: Honoraria. Kapoor:Glaxo Smith Kline: Research Funding; Amgen: Research Funding; Janssen: Research Funding; Cellectar: Consultancy; Takeda: Honoraria, Research Funding; Celgene: Honoraria; Sanofi: Consultancy, Research Funding. Dingli:alexion: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Rigel: Consultancy; Karyopharm: Research Funding. Russell:Imanis: Equity Ownership. Kumar:Janssen: Consultancy, Research Funding; Takeda: Research Funding; Celgene: Consultancy, Research Funding.
Sonidegib (LDE 225) is a selective oral bioavailable antagonist of Smoothened (SMO), a component of the hedgehog signaling pathway.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal