Background
CD38 is highly expressed on MM cells, hence anti-CD38 agents are of interest as a therapeutic approach in MM. The anti-CD38 monoclonal antibody, dara, as mono- or combination therapy, has substantially improved efficacy outcomes in RRMM, including in heavily pretreated pts. However, many pts relapse and new therapeutic approaches are needed, particularly for pts R/R to dara.
TAK-169 is a dimeric fusion protein of an anti-CD38 antibody single chain variable fragment fused to a modified Shiga-like toxin-A subunit. Its unique mechanism of action involves specific binding to CD38+ cells, forced internalization into the target cells, retrograde transport to the cytosol, and irreversible, enzymatic inactivation of target cell ribosomes to cause apoptosis. TAK-169 showed potent, rapid in vitro activity on CD38-expressing cell lines and in vivo efficacy in MM mouse xenograft tumor models with a wide CD38 expression range when administered intravenously (IV) once weekly (QW) or once every 2 weeks (Q2W). Activity was also shown in ex vivo assays from 6 pt bone marrow aspirate (BMA) samples, including from a dara-resistant pt (EC50 5 nM). TAK-169 retained in vitro activity in the presence of dara with modest shifts in EC50 noted at higher dara concentrations. In contrast to dara, TAK-169 has direct tumor cell kill activity that is independent of a pt's immune function status and may therefore be effective in a dara-resistant setting.
Accordingly, this phase 1 study will assess safety, tolerability, preliminary efficacy, pharmacokinetics (PK) and pharmacodynamics (PD) of TAK-169 in RRMM.
Methods
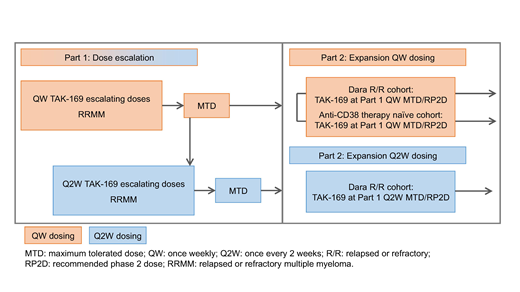
This first-in-human, multicenter, open-label study (NCT04017130; Figure) comprises dose escalation (Part 1) and expansion (Part 2). Eligible pts must be aged ≥18 years with a confirmed diagnosis of MM. Pts enrolled in Part 1 should not be candidates for available therapies known to confer clinical benefit in RRMM. In part 2, pts enrolled should have received ≥3 prior lines of therapy, or ≥2 if one included a PI and IMiD combined. Prior anti-CD38 therapy (including dara) is permitted, except in the Part 2 anti-CD38 therapy-naive expansion cohort.
In Part 1, the primary objective is to assess safety/tolerability of TAK-169 in pts with RRMM, and determine maximum tolerated dose/recommended Phase 2 dose (MTD/RP2D). Secondary objectives include efficacy, PK, PD and immunogenicity of TAK-169. Approximately 60 pts are planned to be enrolled to receive IV TAK-169 QW on Days 1/8/15/22 in 28-day cycles. Dosing will start at 50 μg/kg, with subsequent dose levels of 100, 200, 335, 500, 665 μg/kg. A 2nd Q2W dose escalation cohort may be initiated, starting at the QW MTD. The Bayesian Logistic Regression Model with overdose control will be used for dose escalation cohort 2 and for all subsequent dose escalation cohorts, and other non-DLT-safety, clinical, PK, and PD data will be considered.
In Part 2, the primary objective is preliminary evaluation of TAK-169 clinical activity in RRMM. Secondary objectives include further evaluating safety, efficacy, PK, PD, and immunogenicity of TAK-169. Part 2 will enroll approximately 54 pts into dara RR (QW and Q2W dosing) and anti-CD38 therapy-naïve (QW dosing) cohorts. TAK-169 will be administered at the MTD/RP2D (QW and Q2W) determined in Part 1.
Response will be monitored longitudinally using orthogonal approaches. M-protein levels in serum and urine will be measured each cycle to determine objective responses by International Myeloma Working Group (IMWG) criteria. Minimal residual disease status in pts suspected to be at complete response will be assessed. Circulating free DNA (cfDNA) will be collected from longitudinal plasma samples for disease monitoring. Baseline cytogenetic data will be collected, and TAK-169 efficacy will be assessed in pts with high-risk cytogenetics.
Serial blood samples will be used to characterize PK and PD of TAK-169 after IV administration, and to assess the presence of antidrug antibodies. BMA samples collected at screening will be used to evaluate baseline expression of candidate biomarkers.
Toxicity will be evaluated using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5. Efficacy analyses will use descriptive statistics with a 95% confidence interval and the Kaplan-Meier method. PK parameters and immunogenicity status will be summarized using descriptive statistics.
Recruitment is ongoing.
Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Cornell:Takeda: Consultancy; KaryoPharm: Consultancy. Landgren:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Other: IDMC; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Theradex: Other: IDMC; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ailawadhi:Cellectar: Research Funding; Pharmacyclics: Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy; Amgen: Consultancy, Research Funding; Takeda: Consultancy. Higgins:Molecular Templates, Inc.: Employment, Equity Ownership. Willert:Molecular Templates: Employment. Waltzman:Molecular Templates, Inc.: Employment, Equity Ownership. Lin:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Zhang:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Lublinsky:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dash:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Hanley:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Manoharan:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Leichter:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Ottinger:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Labotka:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Newcomb:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Vorog:Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment.
TAK-169 is a dimeric fusion protein of an anti-CD38 antibody single chain variable fragment fused to a modified Shiga-like toxin-A subunit
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal