Background: Patients with RRMM who have relapsed through multiple prior lines of therapy need novel, effective, targeted agents. B-cell maturation antigen (BCMA) is a cell-surface receptor required for plasma cell survival that is ubiquitously expressed on MM cells, but virtually absent on naïve and memory B cells.
Belantamab mafodotin (GSK2857916) is a BCMA-directed immuno-conjugate with an afucosylated, humanized anti-BCMA monoclonal antibody (mAb) conjugated by a protease-resistant cysteine linker to a microtubule disrupting agent, monomethyl auristatin F (MMAF). Belantamab mafodotin specifically binds to BCMA, eliminating MM cells by a multimodal mechanism including delivering MMAF to BCMA-expressing malignant cells, enhancing antibody-dependent cellular cytotoxicity, and leveraging immunogenic cell death. In an open-label, phase 1 study (DREAMM-1), belantamab mafodotin monotherapy had a manageable safety profile and demonstrated rapid, deep and durable clinical response (60% overall response rate, 14.3 months duration of response) with significant progression free-survival (PFS; 12 months) in patients with heavily pretreated MM. Belantamab mafodotin is being evaluated in clinical trials in various lines of MM therapy, either as monotherapy or in combination.
The DREAMM-5 platform trial is a phase 1/2 study that incorporates an efficient design with 1 master protocol, wherein multiple belantamab mafodotin-containing combinations will be evaluated in separate substudies to identify effective doublet combinations versus a shared belantamab mafodotin monotherapy control arm. Initial substudies will include combinations with the T-cell activating checkpoint mAbs GSK3359609 (an IgG4 inducible T-cell costimulatory [ICOS] agonist antibody that is Fc optimized to selectively enhance T-cell function to enable antitumor responses), GSK3174998 (a humanized wild-type IgG1 anti-OX40 agonistic mAb), and a gamma-secretase inhibitor, Nirogacestat (PF-03084014, SpringWorks Therapeutics). Furthermore, as treatment paradigms evolve, including potential genomic subsets of patients with RRMM and actionable mutations, other control arms may be introduced. The combination agent for evaluation in each substudy will be selected based on scientific rationale and/or results of preclinical experiments with the selected agent in combination with belantamab mafodotin.
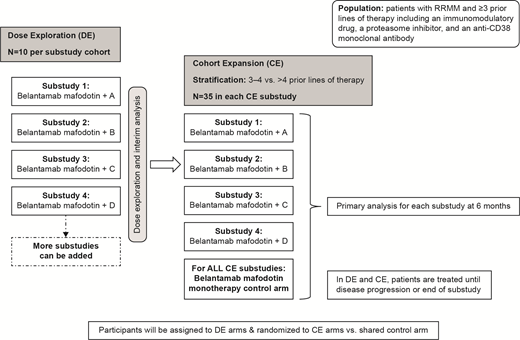
Methods: Patients with RRMM will be eligible if they have received ≥3 prior lines of therapy (consisting of an immunomodulatory drug, proteasome inhibitor, and an anti-CD38 mAb), have measurable disease (as measured by M-protein and free light chain), and acceptable hematologic and vital organ functions. Participants previously treated with an anti-BCMA targeted therapy are eligible except for prior belantamab mafodotin treatment and chimeric antigen receptor (CAR) T-cell therapy within 3 months of screening.
Each substudy will have 2 sequential phases to evaluate safety and efficacy (Figure). The dose exploration (DE) phase will evaluate the safety and tolerability of belantamab mafodotin administered in combination with a partner agent. Each DE phase will consist of multiple dosing cohorts (N≤10 per cohort), 1 cohort per doublet combination. The primary objective is to identify a recommended phase 2 dose for each doublet combination based on safety and preliminary efficacy. Once a substudy is selected for the cohort expansion (CE) phase (N=35 per substudy), the objective is to compare the response rate between the doublet combination and the shared belantamab mafodotin monotherapy control arm. Secondary objectives in CE are to assess AEs, durability of response, PFS, and overall survival. Exploratory objectives include assessment of PK for belantamab mafodotin with each partner agent, bone marrow minimal residual disease status, and plasma soluble BCMA levels as candidate prognostic and predictive biomarkers.
DREAMM-5 is designed to efficiently identify novel, highly effective belantamab mafodotin-containing doublet combinations for evaluation in pivotal trials against current standard-of-care MM agents.
Acknowledgments: Editorial assistance provided by Sarah Hauze, PhD, at Fishawack Indicia Ltd and funded by GSK.
Study funded by GSK (208887); drug linker technology licensed from Seattle Genetics; monoclonal antibody produced using POTELLIGENT Technology licensed from BioWa.
Richardson:Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Biswas:GSK: Employment, Equity Ownership. Holkova:GlaxoSmithKline: Employment, Equity Ownership. Jackson:GlaxoSmithKline: Employment, Equity Ownership. Netherway:GlaxoSmithKline: Employment, Equity Ownership. Bao:GSK: Employment, Equity Ownership. Ferron-Brady:GlaxoSmithKline: Employment, Equity Ownership. Yeakey:GlaxoSmithKline: Employment, Equity Ownership. Shelton:GlaxoSmithKline: Employment, Equity Ownership. Montes De Oca:GlaxoSmithKline: Employment. Ahlers:GlaxoSmithKline: Employment, Equity Ownership. Franco:GlaxoSmithKline: Employment, Equity Ownership. Ballas:GlaxoSmithKline: Employment, Equity Ownership; Bristol-Myers Squibb: Equity Ownership; AstraZeneca: Patents & Royalties: Uncompensated copyrights. Paul:GlaxoSmithKline: Employment, Equity Ownership. Luptakova:GlaxoSmithKline: Employment, Equity Ownership. Gupta:GlaxoSmithKline: Employment, Equity Ownership. Opalinska:GSK: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal