Background:
The treatment indications for venetoclax in CLL are broadening quickly. While the initial approval was for relapsed patients with del 17p (Stilgenbauer S, et al. JCO 2018), it has subsequently been extended to all patients (Seymour J, et al. NEJM 2018, Fischer K, et al. NEJM 2019). The duration of therapy with each approval has evolved as well, from continuing therapy until progression/toxicity to 1 year in front-line CLL. The limited durations of therapy were applied to all patients based on trial design and were not dependent on response to treatment.
Undetectable minimal residual disease (U-MRD) with venetoclax is associated with improved progression-free survival (PFS), both as a single agent and in combination with rituximab (Stilgenbauer S, et al. JCO 2018, Seymour J, et al. NEJM 2018). Early phase data suggest that patients who discontinue venetoclax after achieving MRD negativity can be monitored off therapy and successfully retreated upon relapse (Seymour J, et al, Lancet Onc 2017). However, patients who discontinue therapy with persistent residual disease have a higher incidence of relapse (Kater A, et al. JCO 2019).
Together, these data suggest that the duration of venetoclax therapy should be individualized, based on the depth of response one achieves and not a fixed duration schedule. Therefore, we propose a prospective clinical trial, utilizing MRD assessment with a next generation sequencing (clonoSEQ®) assay to guide clinical decision making in patients with CLL receiving venetoclax-based regimens.
Methods:
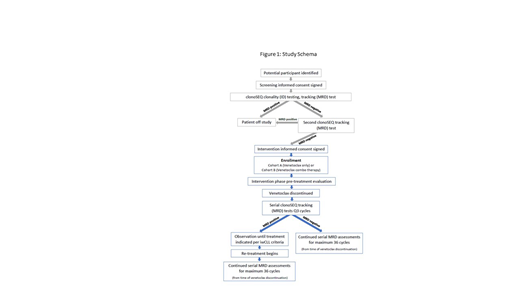
This is a multicenter, phase II clinical stopping trial for 80 venetoclax-treated CLL patients. The clonoSEQ® assay is used to assess MRD, and patients who achieve U-MRD (defined as <10-5) will enter a period of treatment-free observation with serial MRD assessments. Patients will undergo retreatment upon progression of disease requiring therapy per iwCLL criteria (Hallek, Blood 2018).
Patients are eligible if they have CLL/SLL and are currently receiving venetoclax monotherapy or in combination with anti-CD20 (front-line or relapsed). Following informed consent for screening, the ClonoSEQ® ID test will be performed on a sample containing active disease, either fresh (blood or bone marrow) or stored specimens (3-5 bone marrow aspirate slides, 3-5 FFPE slides from a pathologically involved lymph node, or 1 mL involved peripheral blood/bone marrow aspirate). Following ClonoSEQ® ID testing, patients will be evaluated for MRD in the peripheral blood by the ClonoSEQ® MRD assay at two time points, at least 28 days apart.
Patients who have received at least 6 months of venetoclax and achieve U-MRD will sign an intervention informed consent and enter a period of treatment-free observation. Close monitoring with serial MRD assessments on the peripheral blood will occur every three cycles for 36 cycles (1 cycle = 28 days; Figure 1).
Patients who remain MRD undetectable or become MRD positive will continue to be observed until clinical progression warranting therapy by iwCLL criteria. If a patient develops a clinical indication for treatment, they are recommended to be retreated with the same venetoclax-based regimen. Re-treated patients will undergo standard response/toxicity assessments and serial assessment of MRD status every three cycles. Response and MRD assessments for retreated patients will continue for a total of 12 cycles or to the end of 36 cycles from the time of treatment free observation, whichever occurs first.
The primary endpoint of the study is the proportion of patients able to remain off venetoclax-based therapy at 12 cycles following treatment discontinuation. The secondary endpoints include (1) the cumulative incidence of MRD positivity (2) cumulative incidence of retreatment during treatment-free observation (3) PFS and OS as measured from start of treatment-free observation. Should a patient require re-treatment, endpoints of interest include ORR, CR rate, MRD negativity rate, and adverse event profile for venetoclax-based therapy re-treatment. Healthcare outcomes endpoints including economic analysis of cost savings for patients discontinue therapy and quality of life assessment pre- and post-venetoclax discontinuation will be evaluated.
Ujjani:AbbVie: Honoraria, Research Funding; Genentech: Honoraria; Gilead: Consultancy; PCYC: Research Funding; Astrazeneca: Consultancy; Atara: Consultancy; Pharmacyclics: Honoraria. Roeker:AbbVie: Equity Ownership; Abbott Laboratories: Equity Ownership. Shadman:TG Therapeutics: Research Funding; Gilead: Research Funding; Genentech, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy; Sound Biologics: Consultancy; Pharmacyclics: Consultancy, Research Funding; ADC Therapeutics: Consultancy; AbbVIe: Consultancy, Research Funding; Merck: Research Funding; Atara: Consultancy; Verastem: Consultancy; Mustang Biopharma: Research Funding; Bigene: Research Funding; Celgene: Research Funding; Sunesis: Research Funding; Acerta: Research Funding; Emergent: Research Funding. Jacob:Adaptive Biotechnologies: Employment, Other: shareholder. Geyer:Amgen: Research Funding; Dava Oncology: Honoraria. Zelenetz:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mato:Gilead: Research Funding; Acerta: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Celgene: Consultancy; Johnson & Johnson: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; LOXO: Consultancy, Research Funding; DTRM Biopharma: Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal