Ribonucleotide Reductase (RNR) is the enzyme that converts ribonucleotides to deoxyribonucleotides required for DNA replication and repair. RNR consists of two subunits, termed subunit 1 (RRM1) and 2 (RRM2). Imbalance in the regulation of RNR activity and control of dNTPs' pool leads to genomic instability and increases mutation rate. The amount of the enzyme present in the cell and allosteric mechanisms with positive and negative effectors control its activity. 5-Azacytidine (AZA) has been found to be a potent RRM2 inhibitor in acute myeloid leukemia. High expression of RNR is associated with chemoresistance and poorer overall survival in several malignancies and antisense inhibitors that bind to the enzyme mRNA have shown promising results. Moreover, RNR overexpression is a potential mechanism for chemoresistance to nucleoside analogs competing for DNA incorporation. These important roles have made RNR an attractive therapeutic target.
Aim.
We investigated the potential role of RNR as a prognostic factor in patients with MDS and the correlation of bone marrow RNR mRNA levels with treatment response to AZA.
Methods.
Bone marrow samples were collected from patients with MDS at diagnosis. RNA extraction and reverse transcription were performed using standard protocols. A Taqman based real-time PCR was performed on a CFX96 RT-PCR system (Bio-Rad Laboratories, Hercules, CA, USA). For both the housekeeping and target genes, a Taqman primer/probe mix was used according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). RRM1 and RRM2 mRNA levels were expressed as an RRM1-2/beta-actin ratio. A High-Resolution Melt (HRM) analysis for mutation detection of 8 genes of interest in MDS (IDH1, IDH2, SRSF2, SFEB1, UAF1, ASXL2, DNMT3A, BCOR) and quantification of the methylation levels of RRM1/RRM2 promoters were also carried out in a subset of 63 samples. IBM SPSS statistics, version 23.0 (IBM Corporation, North Castle, NY, USA) was used for the analysis of the results.
Results
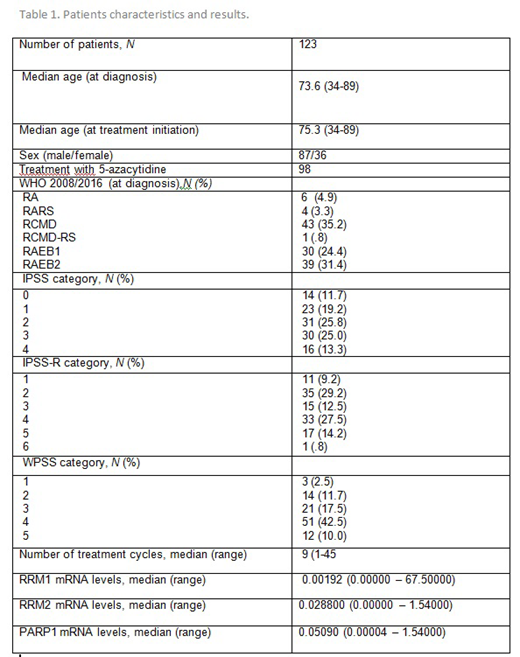
The study included 123 patients diagnosed with MDS per the WHO 2008/2016 classification; 98 of them were treated with AZA. The basic characteristics of the patients are shown in Table 1. The median mRNA levels of RRM1 were 4.2 times higher in non-responders (p=0.019) in comparison to responders. The levels of RRM2 did not differ between the two groups. We found no correlation of any other of the studied factors (MDS type, IPSS, IPSS-R, WPSS, karyotype risk, number of cytopenias, hemoglobin level, WBC and platelet count) to treatment response. The median Overall Survival (OS) of the cohort was 38.6 months. The median survival after treatment with AZA (OST) was 20.4 months. No statistically significant correlations of OS and OST with the levels of RRM1/2 mRNA were found.
A statistically significant correlation was found between splicing mutations and lower RRM1 mRNA levels (p=0.044). They were also correlated with higher median age (79.7 vs 69.7, p=0.005) as well as with lower hemoglobin levels (median 9.4 vs 10.3 g/dL, p=0,021). SF3B1 mutation was found to be correlated with higher RRM2 mRNA levels (median 0.2850 vs 0.0241, p=0.023) and with increased platelet and WBC counts. There was no association between the epigenetic mutations and any of the studied variables.
The methylation status of RRM1 and RRM2 was also correlated with treatment response. Higher methylation percentages of both RRM1 (median 64.3% vs 25.2%, p=0.004) and RRM2 (median 24.5% vs 11.8%, p=0.046) were found in responders. The methylation status was not correlated to the mutation status of the patients. No correlation was found between OS or OST and the methylation or mutation status.
Discussion
MDS patients with low RRM1 levels had a better response to AZA-treatment. Moreover, high RRM1 methylation status was observed to responders, thus making RNR a possible biomarker for predicting success of epigenetic treatment. This is theoretically sound, since inhibition of RR leads to deoxyribonucleotide pool reduction, which is fatal for DNA synthesis and repair. RNR activity is related directly to dNTPs production, therefore AZA-mediated inhibition is likely to cause disturbances in DNA synthesis. Although, patients bearing splicing mutations had low RRM1 levels, SF3B1+ patients did not respond to AZA.
In conclusion, expression of RNR could be used as a prognostic factor for response to AZA treatment and a possible therapeutic target in MDS.
Symeonidis:MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Tekeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kotsianidis:Celgene: Research Funding. Pappa:Novartis: Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene / GenesisPharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Research Funding; Abbvie: Research Funding. Panayiotidis:Bayer: Other: Support of clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal