Background
The inflammatory process plays a pivotal role in the pathogenesis of Myeloproliferative neoplasms (MPN)and immune dysregulation has been well described in this population, who may display both impaired regulatory T cell and CD4/natural killer cell function (Barosi, 2014).This inherent immune incompetence means that infections such as Influenza A (H1N1) may cause significant morbidity, even mortality, for these patients and, in general, the seasonal influenza A vaccination is recommended in immunocompromised MPN patients. To date, knowledge concerning the protective efficacy of the vaccine in this cohort is limited (Ljungman, 2005; Rieger, 2018). The aim of this study was to determine whether patients with MPN mount an adequate protective immunological response to the Influenza A vaccine (2016) when compared with healthy controls. Mass cytometric analysis using a panel of 35 antibodies was employed to assess the immune response generated following Influenza A vaccination by interrogating both innate and adaptive immune cells.
Methods and Results
MPN patients were invited to receive the seasonal Influenza A vaccination as recommended by the WHO in October 2016. Vaccination was administered subcutaneously with inactivated influenza A vaccine (split viron) and peripheral blood samples were collected at both 3- weeks and 3- months post vaccination. A total of 19 patients and 2 HDs were enrolled. The median age of the MPN cohort was 50 years (range 30-78). As regards disease type, 8 patients had Essential Thrombocythaemia, 7 patients Polycythaemia vera and 4 patients Myelofibrosis, of this cohort, 5 were treatment naïve, 6 patients were treated with interferon, 4 with hydroxycarbamide and 4 with a JAK inhibitor.
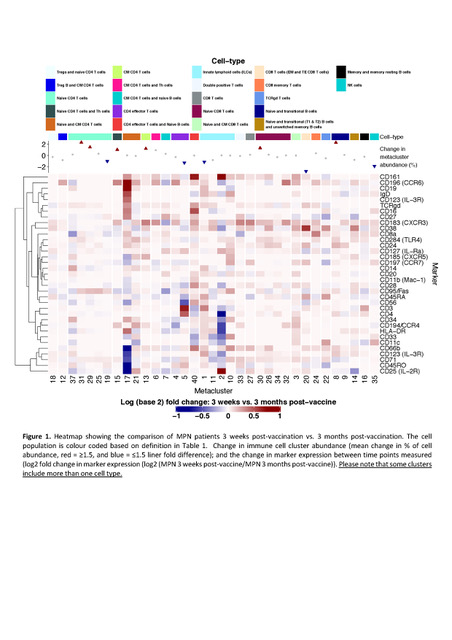
Deep immunophenotyping was performed using a focussed high-dimensional single-cell analysis with Mass Cytometry (CyTOF). Peripheral blood mononuclear cells (PBMCs) were isolated from MPN patients 3 weeks post- (n=16), and matched 3 months' post-vaccine (n=17); and HDs 3 weeks post- (n=2), and 3 months' post-vaccination (n=2). PBMCs were stained with 35 metal-tagged antibodies and analysed using CyTOF. Automated dimension reduction method including viSNE to identify cell populations in combination with FlowSOM clustering were used. The identified cell clusters and the marker expression of each cluster of cells are summarised in figure 1.
In MPN patients, an increase in abundances were identified at 3 months' post-vaccination, in a cluster of central memory (CM) CD4 T cells (p= 0.02); and Innate lymphoid cells (ILCs) (p= 8.98 x 10-4). However, the majority of defined immune cell clusters showed no significant differences in either abundances or marker expression post-vaccination (Figure 1). In contrast, for the HDs, several immune cell clusters were expanded in response to vaccination, including NK cells,CM CD4 T cells,naïve CD8 T cells, and naïve CD4 T cells. In addition, the expression of defining markers were decreased in naïve and CM CD4+T cells (p= 9.16 x 10-3), ILCs (p= 0.02); and naïve and transitional B cells (p= 0.03) between these time points.
Conclusions
Cell mediated immunity plays a central role in defence against the influenza infection. Vaccine mediated protection is dependent upon innate and adaptive subsystems with both T helper and cytotoxic T cell responses demonstrated in healthy individuals (Guthrie, 2004).Our data suggests that while influenza vaccination can elicit some immune response in MPN, it is not as comprehensive as that generated in HDs and the overall resultant change in immune profile of MPN patients is minimal, both in abundances and change in expression of markers across immune cell clusters and throughout both time points measured.
We would also expect to observe a switch from increased proliferation of naïve cell populations to a memory phenotype, providing evidence for an adequate memory type immune response to the vaccine, but this was not the case for the analysed MPN patients. It appears that although the immune systems of MPN patients were able to recognise the Influenza A vaccine, the response was not as comprehensive as the HDs. Given the relatively low numbers of patients we have tested it is not possible to identify a single treatment or disease subtype effect and indeed these findings may not relate to either factor. Further work is required to establish if this phenomenon is treatment related or driven by the underling immune dysregulation.
Dillon:Novartis: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; TEVA: Consultancy, Honoraria. Radia:Blueprint Medicines: Consultancy; Novartis: Consultancy, Speakers Bureau. De Lavallade:BMS: Honoraria, Research Funding, Speakers Bureau; Incyte biosciences: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Harrison:Incyte: Speakers Bureau; AOP: Honoraria; Gilead: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Sierra Oncology: Honoraria; Promedior: Honoraria; Roche: Honoraria; Janssen: Speakers Bureau; Celgene: Honoraria, Speakers Bureau; CTI: Speakers Bureau; Shire: Speakers Bureau. Kordasti:Celgene: Research Funding; Novartis: Research Funding; Boston Biomed: Consultancy; API: Consultancy. McLornan:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal