Introduction: Immunological factors have been associated with deep molecular response (DMR) and treatment-free remission (TFR) in chronic myeloid leukemia (CML). Immune cell functions are restored in patients with DMR and this effect remains after TKI discontinuation. Recently, a multivariable model including CD4+ T cells, PD1+TIM3-CD8+ T cells and neutrophils counts showed ability to predict the likelihood of achieving molecular remission 4.0 (MR4) among other clinical parameters. In this prospective study of TKI discontinuation, we evaluated the expression of two checkpoint receptors, programmed death 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3), in the T cell population of patients who successfully remained in TFR. We also evaluated lymphocyte subpopulations profile before and during TKI discontinuation.
Materials and methods: This study was approved by local ethical committee and registered at the Clinicaltrials.gov (NCT03239886). The inclusion criteria were: age ≥ 18 years, CML chronic phase, treatment with first line imatinib (IM) for at least 36 months, MR4 for at least 12 months confirmed with 3 samples, no previous transplant or resistance to therapy. IM was restarted when loss of major molecular response (MMR) was confirmed by two samples. The primary endpoint was the TFR rate at 24 months. Lymphocyte subpopulations were analyzed in peripheral blood by flow cytometry before discontinuation, IM resumption or in the second year of follow up in patients in TFR. A 6-color flow cytometry panel including CD45, CD3, CD4, CD8, PD-1 and Tim-3 antibodies was also used to study T cell exhaustion phenotype in this population.
Results: Thirty-one patients were included in the study from Dec/2016 to Oct/2017. Median age was 54 years (range 29-95), 58% male, 55% with a low Sokal score, 65% with b3a2 BCR-ABL transcripts, 30% with prior use of interferon and 69% were in MR4.5. The median time of IM therapy was 9.4 years (range 3-14.9) and the median time of sustained MR4 was 6.7 years (range 1.6-10.6). One patient died two months after discontinuation due to acute heart failure (not related to CML). In a median follow up of 29 months (range 21-32), the TFR rate was 56% (95% CI 37-72). Thirteen patients (42%) lost MMR, six (46%) of them after six months of discontinuation. After resumption of IM, twelve patients (92%) recovered MR4; one patient achieved MMR. The median time to recover MMR and MR4 was 1 month and 2.4 months respectively.
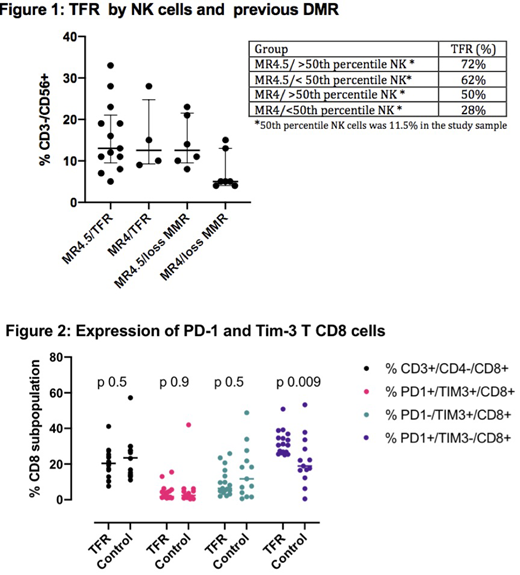
Lymphocytes subpopulations CD3+/CD4+/CD8-, CD3+/CD4-/CD8+ and CD3-/CD56+ (NK cells) proportions were similar among all patients and no variations were observed during the study. The overall median proportion of NK cell was 10.5%, being 13% in the TFR group vs 10% in relapsed patients (p 0.08). Proportion of NK cells and molecular response prior to discontinuation were associated with different rates of TFR as shown in figure 1.
T cell exhaustion was evaluated in the TFR group (n=17) during the second year of follow up. The control group (n=13) were the patients that lost MMR during the study, but their samples were collected after they recovered MMR/MR4 with IM. Patients in TFR had a median proportion of PD-1+Tim3-CD8+ T cells of 30.6% (25.1-50.9) vs 18.9% (0.5-53.3) in the control group (p 0.009). No differences were observed in PD-1+Tim3+CD8+, PD-1-Tim3-CD8+ and in CD4+ T cells populations (figure 2).
Conclusion: In this prospective cohort the median duration of sustained MR4 was above 5 years, as recommended by most guidelines of TKI discontinuation. Patients with only MR4 and lower counts of NK cells had the lowest probability to remain in TFR (28% vs 72%). This data suggests that the depth of molecular response combined with NK cells proportion might be useful to refine selection of patients for discontinuation trials. PD-1+Tim3-CD8+ T cells proportion was higher in patients who were free of TKI compared to those patients who had to resume imatinib. Further studies are needed to evaluate whether the reversion of T cell exhaustion phenotype is mediated by TKI and how this impacts the CD8+ T cells cytotoxicity of patients in TFR.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal