Introduction: The tyrosine kinase inhibitor (TKI) bosutinib is approved for patients with Philadelphia chromosome (Ph)+ chronic myeloid leukemia (CML) resistant/intolerant to prior therapy and newly diagnosed patients in chronic phase (CP).
Methods: The ongoing phase 4 BYOND study (NCT02228382) is further evaluating the efficacy and safety of bosutinib for CML resistant/intolerant to prior TKIs. Patients were administered bosutinib at a starting dose of 500 mg once daily (QD). Primary results were previously reported. Here, we report the efficacy of bosutinib 500 mg QD in patients with Ph+ CP CML and resistance to imatinib (but not to nilotinib or dasatinib) vs patients with resistance to ≥1 second-generation TKI (dasatinib and/or nilotinib), as well as in patients with intolerance to all prior TKIs. Data are reported at ≥1 year after last enrolled patient; 85% of patients had a minimum follow-up of 2 years.
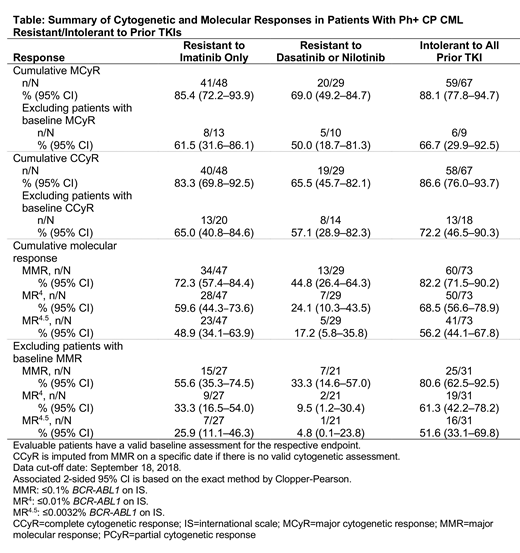
Results: Of 163 patients who received bosutinib, 156 had Ph+ CP CML: 52 had resistance only to imatinib, 31 had resistance to dasatinib/nilotinib, and 73 were intolerant to all prior TKIs. Corresponding median treatment duration (range) was 24.1 (0.2-42.2), 8.9 (0.9-41.6), and 25.3 (0.4-41.9) months, and median dose intensity (range) was 360 (125-500), 431 (195-561) and 292 (80-500) mg/day. In all, 69.2%, 41.9%, and 53.4% of imatinib-resistant, dasatinib/nilotinib-resistant, and TKI-intolerant patients, respectively, were still receiving treatment as of the data cutoff date. The main reason for discontinuation was adverse events (AEs), with 10 (19.2%), 8 (25.8%), and 21 (28.8%) imatinib-resistant, dasatinib/nilotinib-resistant, and TKI-intolerant patients, respectively, discontinuing due to AEs. Corresponding discontinuations due to insufficient response occurred in 2 (3.8%), 5 (16.1%), and 1 (1.4%) patients. No patient experienced on-treatment transformation to advanced phase CML or discontinued treatment due to disease progression. In the evaluable cytogenetic population, cumulative major cytogenetic response (MCyR) rates were 85.4%, 69.0%, and 88.1% in imatinib-resistant, dasatinib/nilotinib-resistant, and TKI-intolerant patients, respectively (Table). The majority of patients, across all cohorts, achieved a complete cytogenetic response (CCyR). In the evaluable molecular population, cumulative major molecular response (MMR) rates were 72.3%, 44.8%, and 82.2% in imatinib-resistant, dasatinib/nilotinib-resistant, and TKI-intolerant patients, respectively; the 50th percentile of the cumulative incidence curve was 5.66 months, not reached and 3.22 months, respectively. Correspondingly, 59.6%, 24.1%, and 68.5% achieved molecular response (MR)4, and 48.9%, 17.2%, and 56.2% achieved MR4.5. In imatinib-resistant, dasatinib/nilotinib-resistant, and TKI-intolerant patients, respectively, Kaplan-Meier estimated overall survival rates (95% confidence interval) were 96.1% (85.2-99.0), 100% (100-100), and 98.6% (90.5-99.8) at 1 year, and 96.1% (85.2-99.0), 92.6% (73.4-98.1), and 97.2% (89.2-99.3) at 2 years with 4, 3, and 3 deaths occurring on study.
Conclusions: Cytogenetic and molecular responses were seen in a high proportion of patients with Ph+ CP CML and TKI-resistance or TKI-intolerance. Response rates were similar between patients with resistance to imatinib and patients who were intolerant to all prior TKIs. Although to a lesser degree, responses were also seen in patients with resistance to second-generation TKIs, including patients achieving MR despite the shorter treatment duration. These results further support bosutinib use for patients with Ph+ CP CML and resistance/intolerance to prior TKIs.
Smith:Agios: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Celgene: Consultancy; Jazz: Consultancy. Brümmendorf:Janssen: Consultancy; Novartis: Consultancy, Research Funding; Ariad: Consultancy; Merck: Consultancy; University Hospital of the RWTH Aachen: Employment; Pfizer: Consultancy, Research Funding. Roboz:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gambacorti-Passerini:Bristol-Meyers Squibb: Consultancy; Pfizer: Honoraria, Research Funding. Charbonnier:Novartis: Consultancy; Pfizer: Consultancy; Incyte: Speakers Bureau. Viquiera:Pfizer: Employment, Equity Ownership. Leip:Pfizer: Employment, Equity Ownership. Giles:Novartis: Consultancy; Epigene Therapeutics Inc: Consultancy, Other: leadership, stock/other ownership ; Actuate Therapeutics Inc: Employment. Ernst:Novartis: Research Funding. Hochhaus:Incyte: Research Funding; MSD: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Novartis: Research Funding. Rosti:BMS: Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal