Chronic myelogenous leukemia (CML) is a malignant myeloproliferative disease characterized by the formation of the BCR-ABL fusion gene. At present, basic studies of the pathogenesis of relapse after stopping tyrosine kinase inhibitors (TKIs) treatment have mainly concentrated on two main aspects: the leukemia stem cells (LSCs) and the tumor microenvironment. However, whether relapse or non-relapse patients who discontinued TKIs therapy, LSCs are still exist. Among a variety of factors that compose the CML microenvironment, myeloid-derived suppressor cells (MDSC) are considered to be a strong contributor to the immunosuppressive tumor microenvironment. Here, we designed the study to investigate the potential relation between tumor cells and MDSC in CML and find risk factors for relapse after discontinuation.
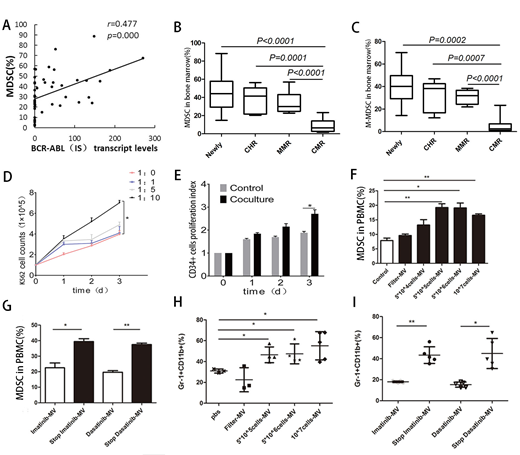
We detected the percentage of MDSC and the BCR-ABL (IS) transcript levels in bone marrow of 50 CML patients in chronic phase at our center. The data indicated that the frequency of MDSC had significant positive correlation with BCR-ABL (IS) transcript levels (Figure 1A). In addition, the counts of MDSC had significant difference at different response stages (Figure 1B), especially the M-MDSC, a subtype of MDSC. The percentage of M-MDSC was significantly higher in patients with newly diagnosed or complete hematological response (CHR) or major molecular response (MMR) compared with those of CML patients obtained complete molecular response (CMR) (Figure 1C). When K562 cells or CD34+ cells were cocultured with M-MDSC at a 1:10 ratio, K562 cells or CD34+ cells proliferated significantly at day 3 (Figure 1D and E). K562 subcutaneous tumor formation in BALB/c node mice confirmed that tumors weight and volume of the coculture group were higher than control.
Then, we further investigated whether tumor cells have an impact on MDSC through microvesicles (MV). After adding K562-MV to peripheral blood mononuclear cells (PBMCs) from healthy donors, MDSC counts appeared significantly elevated in the different K562 cells counts group compared to the control group (Figure 1F).To analyze the roles of K562-MV collected before and after TKIs discontinuation on MDSC, we established a TKIs discontinuation model using the K562 cell, which emulates the cessation of TKIs treatment of CML patients in some extent. The results showed that regardless of the Imatinib or Dasatinib treatment, a significant increase was observed in the proportion of MDSC after TKIs treatment cessation compared with the TKIs treatment groups (Figure 1G). Experiments in vivo also proved K562-MV after different treatments promoted the proliferation of MDSC (Figure 1H and I).
In conclusion, our study introduces the notion of the role of MDSC as mediators in the cross talk between tumor cells and the microenvironment. MDSC would provide a novel and useful model to predict the relapse of CML by establishing a type of new risk stratification system. MDSC could be also act as a promising target in the relapse of CML. In addition, we found a mutual promotion of proliferation of tumor cells and MDSC, this bidirectional interaction results in a vicious cycle by providing a protective niche against immune attacks. Therapeutic interventions modulating this interaction might accelerate the success of treatment-free remission.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal