Background: Fever is a cardinal symptom of cytokine release syndrome (CRS) after CAR T-cell therapy with 84% of patients experiencing fever on the ZUMA-1 trial of axicabtagene ciloleucel (axi-cel). Knowledge of the patterns of fever and associated symptoms may inform the clinical management of these patients.
Methods: We performed a single center retrospective study in 78 patients receiving axi-cel for large B cell lymphoma (LBCL) as of 12/31/2018. We evaluated all the patients who developed fever during lymphodepleting chemotherapy with fludarabine (Flu) and cyclophosphamide (Cy), after CAR T-cell infusion, and after administration of tocilizumab (toci); and analyzed the association of fever with toxicity rates (grade 3+ CRS and neurotoxicity) and efficacy [overall response rates (ORR) and complete response (CR) rate 6 months post CAR T-cell infusion]. Fever was defined per the Lee criteria [equal to or greater than 38 °C], CRS used the modified Lee criteria and neurotoxicity used the CARTOX grading system.
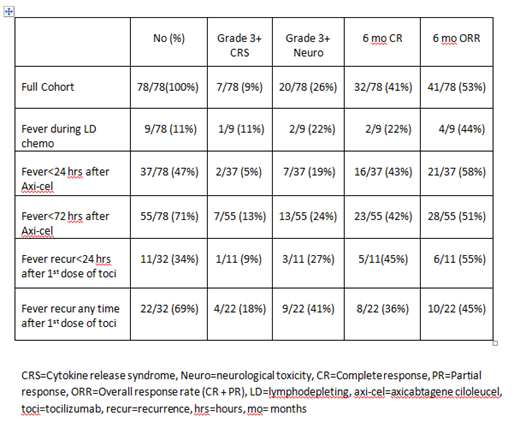
Results: Fever occurred in 71/78 (91%) of patients. Rates of grade 3+ CRS and neurotoxicity were 9% (7/78) and 26% (20/78) respectively. The CR rate at 6 months was 41% (32/78). Toxicities and outcomes in patients with the described fever characteristics are shown in the Table. During lymphodepletion with Flu/Cy, fever was observed in 11% (9/78) of patients. Fever occurred within 24 hours of axi-cel infusion in 47% (37/78) and within 72 hours of axi-cel infusion in 71% (55/78) of the patients. In total, 41% (32/78) of patients were treated with anti-IL6R therapy (tocilizumab; toci) for CAR T toxicity. After the first dose of toci, fever recurred in 69% of patients (22/32), of which 34% (11/32) experienced fever recurrence within 24 hours of toci infusion.
Conclusions: This is the first study to our knowledge that describes in detail the characteristics of fever after CAR T-cell therapy with axi-cel. Fever was common and occurred in 71% of the patients within 72 hours of axi-cel infusion. When toci was used, fever recurred in a majority of patients (69%) and in 1/3 of patients the fever recurred within 24 hours of toci infusion. These descriptive data may be used by clinicians to inform their expectations of fever occurring after treatment with axi-cel and/or toci.
Bachmeier:Kite/Gilead: Speakers Bureau. Chavez:Genentech: Speakers Bureau; Kite Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals, Inc.: Speakers Bureau. Shah:AstraZeneca: Honoraria; Novartis: Honoraria; Spectrum/Astrotech: Honoraria; Adaptive Biotechnologies: Honoraria; Pharmacyclics: Honoraria; Jazz Pharmaceuticals: Research Funding; Incyte: Research Funding; Kite/Gilead: Honoraria; Celgene/Juno: Honoraria. Pinilla Ibarz:Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Sanofi: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Bayer: Speakers Bureau; TG Therapeutics: Consultancy; Teva: Consultancy; Janssen: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau. Nishihori:Novartis: Research Funding; Karyopharm: Research Funding. Lazaryan:Kadmon: Consultancy. Davila:Bellicum: Consultancy; Anixa: Consultancy; GlaxoSmithKline: Consultancy; Precision Biosciences: Consultancy; Novartis: Research Funding; Adaptive: Consultancy; Celgene: Research Funding; Atara: Research Funding. Locke:Cellular BioMedicine Group Inc.: Consultancy; Kite: Other: Scientific Advisor; Novartis: Other: Scientific Advisor. Jain:Kite/Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal