Introduction. Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphomas (NHL). The use of chemoimmunotherapy (R-CHOP) in DLBCL has improved Overall Survival (OS). However, there are among 45-55% of patients who will die due to relapse, progression (refractoriness) or toxicity to treatment. Genomic classifications are difficult to use in clinical practice, especially in lain America, due to the need of trained personnel and cost. So, we continue using the International Prognostic Index (IPI) and its variations (e.g. revised-IPI, NCCN-IPI) for prognostic purposes. However, other biological variables have been reported to be prognostic, such as serum beta-2-microglobulin (B2M), lymphocyte/monocyte ratio (LMR), neutrophil/lymphocyte ratio (NLR), platelets/lymphocyte ratio (PLR) and serum albumin (SA). These factors have been associated with burden of disease, inflammation and nutritional status.
Aim. Therefore, we performed a retrospective analysis of the databases of two Latin American groups to determine which biological variable is the most powerful factor prognostic of OS.
Methods. A total of 1,250 patients were analyzed from two databases [(Grupo de Estudio para el Linfoma Mexicano (GELMEX) and the Grupo de Estudio para el Linfoma Latino Americano (GELL)], where 525 patients met the following inclusion criteria: DLBCL diagnosis by immunochemistry; complete data on absolute lymphocyte, absolute monocyte, absolute neutrophil and absolute platelet count, serum B2M and SA; and complete data on traditional variables for calculating risk groups for IPI, NCCN-IPI & R-IPI. The LMR, NLR and PLR was obtained. We evaluated the AUC of the biological variables and the differences among each one of them (including cut-off). Kaplan-Meier curves (KMC) were estimated and subsequently, the inference of OS was evaluated by Hazard Ratio (HR) Cox-regression in univariate/multivariate analyses by forward model. All variables with p<0.05 were considered independent variable for the multivariate analysis.
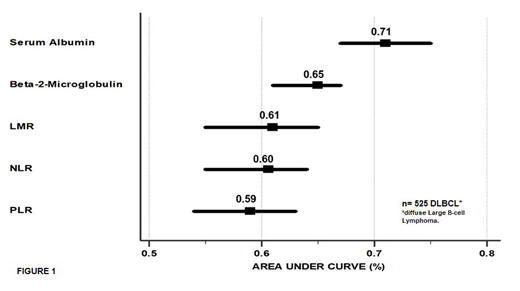
Outcomes: Clinical characteristics of the cohort (n=525) were as follows: median follow-up time was 32 months (range: 0.2-132), 52% were male, 60% had more than 60 y/o (≥75 y/o, 21%), one third (32%) had a bad performance status (ECOG ≥2), 63% had advanced stage (III-IV), 49% had high serum lactate dehydrogenase (LDH; ratio 1.1-2.9, 41%; ratio ≥3, 8%), 21% had ≥2 extranodal sites involved (28%, NCCN-specific sites) and 43% had bulky disease. SA had the best AUC comparing with other variables (see figure 1). 5-y OS rates by SA (>3.2 mg/dL vs. ≤3.2 mg/dL) were 72% vs 34% (Log Rank p<0.0001), by B2M (<4 µg/dl vs. ≥4 µg/dL) were 68% vs. 45% (p<0.0001), by LMR (≥2.2 vs. <2.2) were 67% vs. 47% (p<0.0001), by NLR (<4 vs. ≥4) were 70% vs. 52% (p<0.0001) and by PLR (<475 vs. ≥475) were 65% vs. 44% (p<0.0001). In the univariate Cox regression analysis, SA ≤3.2 mg/dL was associated with HR 3.41 (CI95% 2.47-4.64), B2M ≥4 µg/dL with HR 2.13 (CI95% 1.57-2.88), LMR <2.2 with HR 2.11 (CI95% 1.56-2.87), NLR ≥4 with HR 1.92 (CI95% 1.45-2.55), and PLR ≥475 with HR 2.17 (CI95% 1.52-3.1)]. The multivariate Cox regression analyses (Forward model) showed that SA ≤3.2 mg/dL, B2M ≥4 µg/dL and high NLR ≥4 retained their prognostic value for worse OS (HR 2.87, CI95% 2.1-3.90; p=<0.0001; HR 1.40, CI95 1.02-1.94; p=0.036; and HR 1.43, CI95% 1.06-1.92; p=0.01, respectively). SA ≤3.2 mg/dL was the only independent factor associated with worse OS when adjusted by IPI (HR 2.53, CI95% 1.75-3.67; p<0.0001), NCCN-IPI (HR 2.51, CI95%1.81-3.5; p<0.0001) and R-IPI (HR 3.12, CI95%2.34-4.16; p<0.0001).
Conclusion: Low SA emerges as an easy-to-use, important biological factor prognostic of worse survival, independent of the IPI, R-IPI and NCCN-IPI scores, in Latin American patients with DLBCL treated with chemoimmunotherapy.
M:Roche-Mexico: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Merck-Sharp-Dome: Speakers Bureau. Ramirez-Ibarguen:Roche-Mexico: Consultancy, Speakers Bureau. Peña:Novartis: Other: Congress inscription and flights; Tecnofarma: Other: Congress inscription and flights; Roche: Other: Congress inscription and flights; Biotoscana: Other: Congress inscription and flights; Janssen: Other: Congress inscription and flights; Pfizer: Membership on an entity's Board of Directors or advisory committees. Paredes:Tecnofarma: Honoraria. Gomez-Almaguer:Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Teva: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Abello:Takeda: Other: Participation in advisory board meeting. Tena:Roche-Mexico: Employment. Castillo:Abbvie: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal