The development of chimeric antigen receptor T cell (CAR T) therapy has revolutionized the treatment of relapsed refractory diffuse large b-cell lymphoma (DLBCL). However, its impact in vulnerable older patients, especially those with multi-morbidity and functional limitations, has not been explored. Moreover, the Centers for Medicare & Medicaid Services (CMS) has recently proposed Coverage with Evidence Development for CAR T, emphasizing the need for evidence in older patients.
We retrospectively examined outcomes of older patients referred for commercial CAR T products, axicabtagene ciloleucel and tisagenlecleucel, at our institution from January 2018 to March 2019. Forty-two consecutive older patients (≥65yo) were included in the analysis of post-relapse (last documented relapse or refractory state) overall survival (PR-OS) accounting for time of CAR T entry. Geriatric assessment, including comorbidity, basic and instrumental activities of daily living, prior falls, and weight loss, was performed either by a geriatrician prior to admission, or by interdisciplinary clinical staff on the day of admission. In parallel, we compared the safety and toxicities of CAR T between older (≥65yo, n=24) and younger (<65yo, n=25) patients.
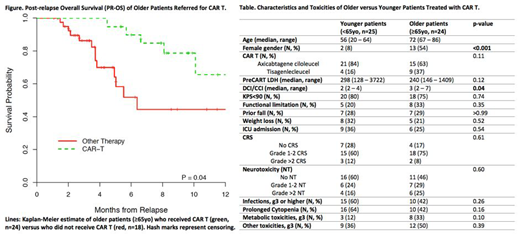
Among the 42 patients ≥65yo, 18 did not receive CAR T due to clinical ineligibility and/or death during the pre-requisite clinical evaluation. Their gender distribution, comorbidity burden, measured by Deyo/Charlson Comorbidity Index (DCI/CCI), and Karnofsky Performance Status (KPS) were comparable to the 24 older patients who received a CAR T product. With a median follow-up of 291 days (range 162 - 572) for survivors, the PR-OS favored the group of older patients who had received CAR T with estimated 1-year PR-OS of 0.67 (95% CI: 0.43, 0.99) versus 0.44 (95% CI: 0.27, 0.75) for patients who did not receive CAR T (p=0.04) (Figure).
We next compared the safety and toxicity profiles among older (≥65yo, n=24) versus younger patients (<65yo, n=25) who received a CAR T. Baseline characteristics were similar among the two groups including: KPS, the prevalence of functional impairment, prior fall, and weight loss, and pre-treatment tumor burden measured by LDH (Table). The older group had more females (p<0.001) and higher comorbidity burden measured by DCI/CCI (p=0.04) (Table). Numerically more younger patients (84%) received axicabtagene ciloleucel compared to tisagenlecleucel versus older patients (63%; p=0.11). Importantly, the two groups had similar incidences of cytokine release syndrome (CRS) and neurotoxicity (NT) of all grades (Table). We also examined the incidence of grade 3-4 hematologic and non-hematologic toxicities by CTCAE v5.0 and found that numerically, older patients appeared to have less infection and cytopenia, and more metabolic and other toxicities (Table). In addition, the rate of Intensive Care Unit admission was similar. At the time of last follow-up, we observed only 1 treatment-related death, a 69-year-old female with a history of prior allogeneic hematopoietic cell transplantation who died of influenza pneumonia 129 days after CAR T infusion.
Although limited by small sample size, retrospective design, and possible patient selection bias regarding disease biology, our results highlight potential benefits of CAR T in selected older patients even with functional limitation, multi-morbidity, and significant tumor burden; and the lack of excessive CRS, NT, and other high-grade toxicities. These findings extend beyond published results of older patients in ZUMA-1 and JULIET trials, and support that, with meticulous management of CAR T toxicities, older patients should not be excluded from CAR T based on chronologic age alone. Detailed geriatric assessment and correlation with toxicities should allow better selection of older adults who could benefit from this curative treatment. In addition, the biology of CAR T response in older adults may warrant additional investigation in the context of aging-associated changes in the immune system.
Batlevi:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Giralt:Jazz Pharmaceuticals: Consultancy; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Actinium: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Kite: Consultancy. Noy:Medscape: Honoraria; Prime Oncology: Honoraria; NIH: Research Funding; Janssen: Consultancy; Pharamcyclics: Research Funding; Raphael Pharma: Research Funding. Palomba:Noble Insights: Consultancy; Hemedicus: Speakers Bureau; Merck & Co Inc.: Consultancy; Seres Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Evelo: Equity Ownership; MSK (IP for Juno and Seres): Patents & Royalties. Santomasso:Kite/Gilead: Consultancy; Novartis: Consultancy; Juno/Celgene: Consultancy. Sauter:Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy; GSK: Consultancy. Scordo:Angiocrine Bioscience, Inc.: Consultancy; McKinsey & Company: Consultancy. Shah:Janssen Pharmaceutica: Research Funding; Amgen: Research Funding. Perales:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Medigene: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; NexImmune: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyte/Gilead: Research Funding; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal