Background: The DIAL study is testing the efficacy of dual immunomodulation in patients with advanced aggressive B cell non-Hodgkin lymphoma (B-NHL). Sponsored by the Cancer Therapy Evaluation Program (CTEP), the trial combines the use of a programmed cell death protein 1 (PD-1) inhibitor (nivolumab) with an agonist of the CD27 receptor (varlilumab) in a randomized phase 2 design. CD27, a co-stimulatory receptor, regulates T cell activation in the context of T cell receptor (TCR) engagement through interaction with CD70. T cell exhaustion plays a major role in immune evasion in B-NHL. Varlilumab is an agonistic IgG1 monoclonal antibody that recognizes CD27 leading to prevention or reversal of exhaustion in pre-clinical models. Varlilumab also demonstrates direct anti-tumoral activity in xenograft models of human lymphoma cell lines via antibody-dependent cell-mediated cytotoxicity. Phase 1 data supports the safety and tolerability of single-agent varlilumab in advanced hematologic malignancies (NCT01460134). We hypothesize that CD27 activation synergizes with PD-1 inhibition resulting in a superior anti-lymphoma effect compared to PD-1 blockade alone. The study will also evaluate the effect of these agents on tumor and immune cells using immunohistochemistry (IHC), mass cytometry (CyTOF), multiplex ELISA, imaging mass cytometry (IMC), and whole exome sequencing.
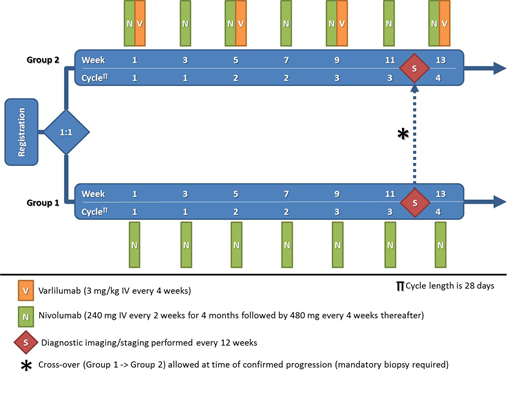
Methods: The trial is enrolling patients with advanced aggressive B-NHL. Standard inclusion criteria and prior treatment with at least 2 lines of standard therapy are required. Prior autologous stem cell transplant and/or chimeric antigen receptor (CAR) T cell therapy is allowed. Patients with active CNS disease are excluded. Eligible patients are randomized to treatment with single-agent nivolumab (group 1) or dual immunotherapy with nivolumab and varlilumab. Group 1 is allowed to cross-over at the time of progression. Nivolumab will be administered intravenously (IV) every 2 weeks (240 mg) for 4 months followed by monthly dosing thereafter (480 mg). Varlilumab will be given IV every 4 weeks (3 mg/kg). Response assessment will be done by PET-CT scan every 12 weeks. Primary outcome is overall response rate (ORR) according to the LYRIC criteria. The trial will enroll 48 patients per arm, allowing 80% power to detect at least 20% increase in ORR in the experimental arm (group 2) assuming a 25% ORR in the control arm (group 1). The trial is registered (NCT03038672) and open to participation to members of the Experimental Therapeutics Clinical Trials Network (ETCTN) and Early Drug Development Opportunity Program (EDDOP).
Tun:BMS: Research Funding; Celgene: Research Funding; Curis: Research Funding; TG Therapeutics: Research Funding; Mundi-pharma: Research Funding; DTRM Biopharma: Research Funding. Bartlett:Pharmacyclics: Research Funding; Merck: Research Funding; Incyte: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Millennium: Research Funding; Kite Pharma: Research Funding; Janssen: Research Funding; Immune Design: Research Funding; Affimed: Research Funding; Autolus: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Forty Seven: Research Funding; Genentech, Inc.: Research Funding; Gilead: Research Funding. Kline:Merck: Honoraria; Merck: Research Funding. Awan:Janssen: Consultancy; Gilead: Consultancy; Sunesis: Consultancy; Genentech: Consultancy; Abbvie: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Speakers Bureau. Lazaryan:Kadmon: Consultancy. Ansell:Affimed: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Affimed: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Trillium: Research Funding. Diefenbach:Bristol-Myers Squibb: Consultancy, Research Funding; Denovo: Research Funding; Genentech: Consultancy, Research Funding; Incyte: Research Funding; LAM Therapeutics: Research Funding; MEI: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Trillium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal