Introduction
Patients with relapsed/refractory Hodgkin lymphoma (R/R HL) experience high response rates upon anti-PD1 therapy. In these patients, the optimal duration of treatment and the risk of relapse after anti-PD1 discontinuation are unknown. Furthermore, the efficacy of anti-PD1 re-treatment in patients who relapse after anti-PD1 discontinuation remains to be determined. Here, we investigated the risk of relapse in patients who responded to anti-PD1 therapy and discontinued the treatment, as well as the efficacy of anti-PD1 re-treatment in patients who relapsed after anti-PD1 discontinuation.
Methods
We retrospectively analyzed patients with R/R HL who responded to anti-PD1 monotherapy (concomitant radiotherapy was permitted) and discontinued the treatment either because of unacceptable toxicity or prolonged remission (based on the clinician's decision). Patients who discontinued because of relapse/progression or underwent consolidation with allogenic stem cell transplantation [alloSCT] were not included. A random forest machine-learning algorithm was trained to predict relapse using 14 candidate biomarkers. Finally, we analyzed the outcome of patients who relapsed after anti-PD1 discontinuation and their response to anti-PD1 re-treatment.
Results
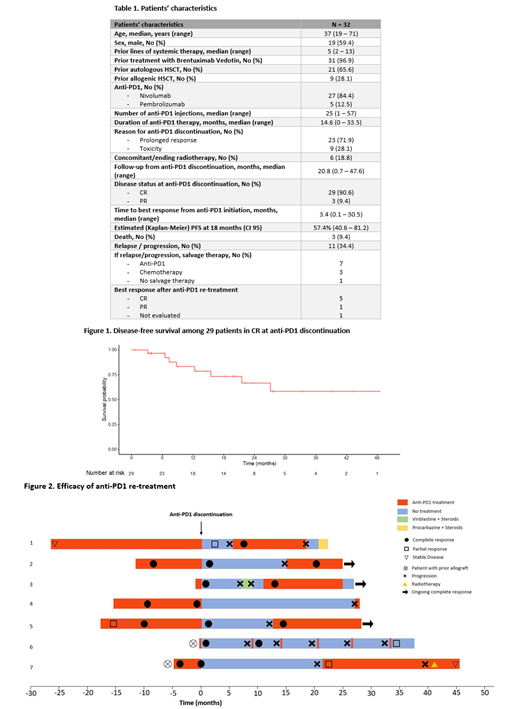
We included 32 patients from 13 Centers in France, Portugal and Belgium. Patients' characteristics are summarized in Table 1. At the time of anti-PD1 discontinuation, patients had received either nivolumab (N=27, 84.4%) or pembrolizumab (N=5, 12.5%) for a median duration of 14.6 (range, 0-33.5) months. Patients discontinued anti-PD1 treatment either because of prolonged remission (N=23, 71.9%) or unacceptable toxicity (N=9, 28.1%). Most patients were in CR (N=29, 90.1%) at the time of anti-PD1 discontinuation.
After a median follow-up of 20.8 months (range, 0.7-47.6) from anti-PD1 discontinuation, 21 (65.6%) patients had not relapsed/progressed. All 3 patients who were in PR at the time of anti-PD1 discontinuation had relapsed. Among the 29 patients who were in CR at the time of anti-PD1 discontinuation, the estimated disease-free survival was 64.3% (CI 95, 46.6-88.7%) at 24 months (Figure 1). Three patients died: two from disease progression and one from severe GVHD while in CR. Interestingly, 4 patients remain in CR more than 3 years after anti-PD1 discontinuation although these patients had received only short courses of anti-PD1 (<6 months). One of them received a single dose of nivolumab for a relapse post-alloSCT and remains disease-free 47.6 months later. Using a testing set of 25 patients, the machine-learning algorithm predicted an increased risk of relapse at 12 months based on three main patients characteristics: the absence of complete metabolic response at the end of anti-PD1 treatment, prolonged time to achieve best overall response, and older age.
Among the 11 patients who relapsed, 7 were re-treated with (the same) anti-PD1 (Figure 2). Five achieved a CR, 1 achieved a PR and one patient has not been evaluated yet (but is in clinical response).
Conclusion
A significant proportion of patients experience prolonged remissions after anti-PD1 discontinuation and thus might be cured. Using a machine-learning algorithm, we identified biomarkers capable of predicting the risk of relapse after anti-PD1 discontinuation. These biomarkers are currently being validated in an independent set of patients. Finally, among patients who relapse after anti-PD1 discontinuation, re-treatment with anti-PD1 appears to be efficient.
Manson:Bristol Myers Squibb: Honoraria. Brice:Takeda France: Consultancy, Honoraria; Millennium Takeda: Research Funding; BMS: Honoraria. Herbaux:Janssen: Honoraria; BMS: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; Gilead: Honoraria. Silva:Abbvie Inc: Consultancy; Celgene: Consultancy; Gilead Sciences: Consultancy, Research Funding; Janssen Cilag: Consultancy; Roche: Consultancy. Stamatoulas Bastard:Celgene: Honoraria; Takeda: Consultancy. Houot:Bristol Myers Squibb: Honoraria; Merck Sharp Dohme: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal