Background: Histone deacetylase inhibitors (HDACi) and combination chemotherapy are independently used to treat relapsed/refractory (R/R) lymphoma. In vitro studies suggest that the addition of HDACi to platinum-based chemotherapy is synergistic. We conducted a phase I study of the combination of romidepsin, gemcitabine, oxaliplatin and dexamethasone in R/R lymphomas with an expansion cohort in T-cell lymphomas. Here we present the initial efficacy results. The maximum tolerated dose (MTD) determination was presented at ASH 2018.
Methods: The safety and tolerability of the combination of romidepsin, gemcitabine, oxaliplatin, and dexamethasone was assessed in this phase I study to determine the MTD with a 9 pt cohort expansion at the MTD. The treatment schedule included: gemcitabine 1000 mg/m2 (day 1), oxaliplatin 100 mg/m2 (day 1); romidepsin (day 2), dexamethasone 20 mg (days 1-4) and pegfilgrastim 6 mg (day 3) of a 21-day cycle. A 3+3 dose escalation was followed with 3 dose levels (DL) of romidepsin: 1) 8 mg/m2, 2) 10 mg/m2, 3) 12 mg/m2. The study originally included romidepsin administration on days 2 and 8 but due to prolonged cytopenias, the day 8 dose was eliminated after the first 6 pts. Dose-limiting toxicity (DLT) was defined in cycle 1 as ≥ gr3 non-hematologic toxicity, any gr4 hematologic toxicity, gr≥ 3 related laboratory changes not responsive to supportive measures, and gr≥2 toxicity resulting in a >14 day treatment delay. Patients could be treated until progression, intolerance, or response adequate to allow autologous or allogeneic transplantation, to a maximum of 8 cycles. Those in complete remission at restaging could limit treatment to 2 cycles beyond complete response.
Results: 24 patients with R/R lymphoma (10 PTCL-NOS, 7 AITL, 6 DLBCL, 1 ALCL) were enrolled with all patients evaluable for toxicity and 23 for response (1 AITL pursued supportive care without progression in cycle 1). The median age was 70 years (range 53-81) with 50% men. The median number of prior therapies was 2 (range: 1-4).
DL 2 was the MTD. There was 1 DLT in 6 pts treated in DL 1 (pneumonia); 1 DLT in 6 pts treated in DL 2 (bleeding); and 2 DLTs in 3 pts treated in DL 3 (neutropenic fever, grade 4 thrombocytopenia). SAEs included hospitalizations for pneumonia (1), nausea/vomiting (1), fever (1), tumor lysis (1), eosinophilic pneumonia (1), febrile neutropenia (1), post-procedural bleeding (1) and for complications of disease progression (7). Other grade 3-4 treatment related toxicities included thrombocytopenia (79%), neutropenia (21%), anemia (13%), hypophosphatemia (21%), hyperglycemia (8%), hypoglycemia (8%), hypokalemia (8%), diarrhea (4%), peripheral neuropathy (4%), and hypernatremia (4%).
The overall response rate (ORR) was 52% (12/23) with complete response (CR) rate 43% (10/23) and the partial response (PR) rate 9% (2/23). At the MTD, ORR was 53% (8/15) and CR 40% (6/15). In patients with TCL, ORR was 58% (10/17) and CR 47% (8/17). CRs were seen in AITL (6/6), PTCL-NOS (2/10) and DLBCL (2/6). PR was seen in PTCL-NOS (2/10). CRs were since in 3 pts refractory to their most recent therapy.
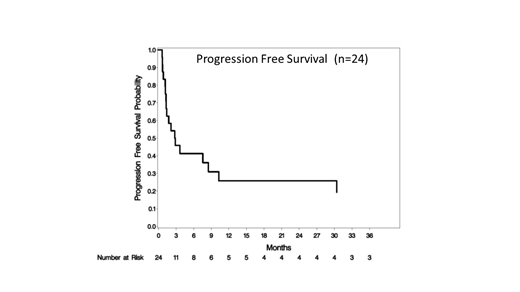
The median progression free survival (mPFS) for all pts was 2.8 months (95% CI: 1.3-10.3) and median overall survival (mOS) was 10.4 months (95% CI: 2.8-33.4) and 10.4 months (95% CI: 1.7-N/A) and 3.3 months (95% CI: 1.2-30.4) respectively in TCL. The median duration of response for all patients was 5.8 months (range: 0.7 - 45.1). At the MTD, mPFS and mOS were 7.6 months (95% CI: 1.2-10.3) and 10.4 (95% CI: 1.7-20.8). 4 patients proceeded to transplant after study treatment (2 allogeneic and 2 autologous) and were censored at transplant. The median time to response was 6.0 weeks. Of others, who achieved a complete response: 2 remain in CR at 18.6 (PTCL) and 37.6 (AITL) months. Three others progressed after >6 months: 45.1 mo (DLBCL), 29.5 mo (AITL), 9.0 mo (AITL).
Conclusions: MTD was identified as romidepsin 10 mg/m2 in combination with gemcitabine 1000 mg/m2, oxaliplatin 100 mg/m2, and dexamethasone 20 mg. There were no unexpected toxicities. The ORR and CR rates of this regimen are promising, particularly in AITL where 6/6 patients had a CR. Durable responses were seen in AITL, PTCL-NOS and DLBCL. Four patients have had responses for >12 months, 4 proceeded to transplant, and 2 remissions are ongoing. Given the durable complete remissions seen in this difficult to treat patient population, this regimen warrants further study.
Mehta-Shah:Verastem Pharmaceuticals: Research Funding; Innate Pharmaceuticals: Research Funding; Kiowa Hakka Kirin: Consultancy; Celgene: Research Funding; Roche/Genentech: Research Funding; Bristol Myers Squibb: Research Funding. Riedell:Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Bayer: Honoraria, Speakers Bureau; Novartis: Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Bartlett:Pharmacyclics: Research Funding; Bristol-Myers Squibb: Research Funding; Forty Seven: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Pfizer: Research Funding; Janssen: Research Funding; Genenetech: Research Funding; Affimed: Research Funding; Celgene: Research Funding; Gilead: Research Funding; Immune Design: Research Funding; Seattle Genetics: Research Funding; Millenium: Research Funding; Merck: Research Funding. Cashen:Celgene: Other: Speaker's Bureau; Seattle Genetics: Other: Speaker's Bureau; Novartis: Other: Speaker's Bureau. Kahl:ADC Therapeutics: Consultancy, Research Funding; BeiGene: Consultancy; TG Therapeutics: Consultancy; Seattle Genetics: Consultancy. Fehniger:Cyto-Sen Therapeutics: Consultancy; Horizon Pharma PLC: Other: Consultancy (Spouse). Carson:Flatiron Health, Inc., which is an independent subsidiary of the Roche Group: Employment, Research Funding; Roche: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal