Background: There is a large unmet need for relapsed/refractory T cell lymphoma. For patients (pts) with recurrent disease, the efficacy of salvage and autologous stem cell transplant (if feasible) is moderate and the 3-year overall survival is less than 30%.
Romidepsin is a histone deacetylase inhibitor (HDACi) that is FDA approved for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL) and cutaneous T-cell lymphoma. Immune checkpoint blockade (CPB) of PD1/PDL-1 has been successful in many malignancies. In a recent report, PDL1 expression was observed 5-17% of tumor cells and 43%-57% in stromal histiocytes in PTCL-NOS or AITL. A phase II single agent study of the PD1 monoclonal antibody (mAB) pembrolizumab in PTCL demonstrated modest responses (Barta et.al). In addition, PTCL often carries multiple gene mutations in epigenetic modifier genes (TET2, IDH2, DNMT3A) and others (RHOA, FYN) that may impair immunogenicity and promote immune escape. This was the rationale for combining HDACi and CPB. We report this single center, single arm, nonrandomized trial of pembrolizumab in combination with romidepsin in subjects with relapsed or refractory PTCL that have failed to achieve a complete response or progressed after at least one systemic treatment regimen (NCT03278782).
METHODS: This is a phase I/II study to examine the safety and efficacy of pembrolizumab 200 mg fixed dose administered every 3 weeks (Q3W) in combination with romidepsin. The safety of the combination was tested in the lead-in phase I study with a strategy of dose de-escalation, keeping the starting dose of romidepsin 14 mg/m2 on days 1 and 8. This eventually became the dosing for the phase II study. Adverse events were monitored throughout the trial and graded in severity according to the guidelines outlined in the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The primary objectives of the trial are to determine safety, tolerability, and overall response rate (ORR) of the combination. Secondary objectives include further analysis of various efficacy parameters [complete remission rate (CRR), progression free survival (PFS), overall survival(OS) and duration of response (DOR)]. Response assessment was performed utilizing Lugano Revised Response Criteria, and PD-L1 expression assessed by immunohistochemistry and exploratory analysis of targeted Next Generation Sequencing in the primary lymphoma tissue. Changes in peripheral blood T-cell repertoire were assessed primarily by flow cytometry.
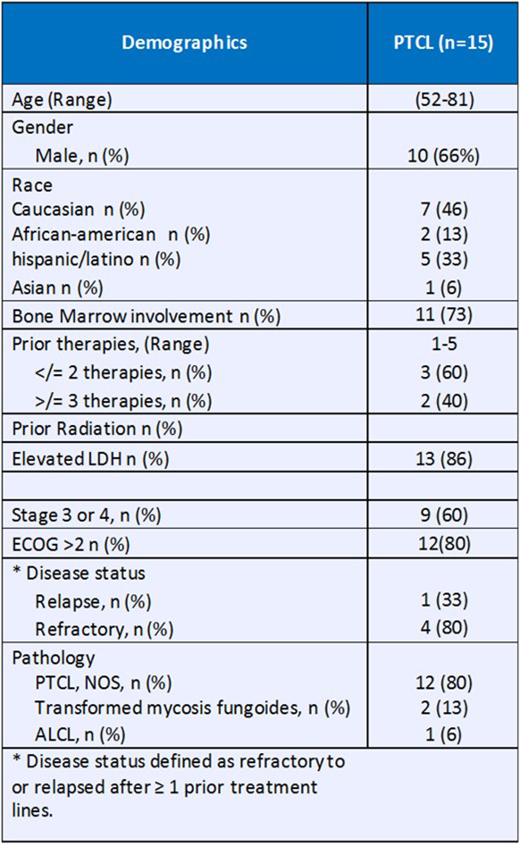
RESULTS: 15 pts were evaluable for the primary endpoint (demographics in figure 1). The phase I part was expanded from 3 to 6 pts, because of a dose limiting toxicity (DLT) of hypotension and renal insufficiency which resolved. The phase II is ongoing with 9 pts enrolled. The overall response rate was 44%; 3 pts. achieved a complete response and 2 achieved PR. The median follow-up was 6 months (3 weeks- 12 months). The 3 complete responders remain in remission > 10 months. One patient with refractory ALCL who achieved CR after 3 cycles is now 6 months post Haplo-identical transplant. Nausea and vomiting was the most common adverse event (Grade 1/2-). The most common ≥ grade 3 treatment-emergent adverse events were Nausea/vomiting and fatigue ( n =1 and n= 2). Two patients experienced hyperprogression within the first 10 days after the treatment. Correlative studies including NGS, expression of PD-L1, CD4+ T lymphocytes counts are in progress.
CONCLUSIONS: The combination of Romidepsin and Pembrolizumab has demonstrated acceptable safety and early encouraging results.
Iyer:Spectrum: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Trillium Therapeutics: Research Funding; Rhizen: Research Funding. Neelapu:BMS: Research Funding; Allogene: Consultancy; Acerta: Research Funding; Incyte: Consultancy; Cell Medica: Consultancy; Pfizer: Consultancy; Unum Therapeutics: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Precision Biosciences: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Karus: Research Funding; Cellectis: Research Funding; Poseida: Research Funding. Nieto:Affimed: Research Funding; Novartis: Research Funding; Astra-Zeneca: Research Funding; Affimed: Consultancy. Westin:Genentech: Other: Advisory Board, Research Funding; Novartis: Other: Advisory Board, Research Funding; MorphoSys: Other: Advisory Board; Juno: Other: Advisory Board; Janssen: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; Unum: Research Funding; 47 Inc: Research Funding; Kite: Other: Advisory Board, Research Funding; Curis: Other: Advisory Board, Research Funding. Parmar:Cellenkos Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding. Fowler:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nastoupil:Bayer: Honoraria; Gilead: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Spectrum: Honoraria; Novartis: Honoraria; TG Therapeutics: Honoraria, Research Funding. Duvic:Janssen Pharmaceuticals (div of Johnson & Johnson): Speakers Bureau; PleXus Communications: Speakers Bureau; Therakos: Speakers Bureau; Eisai: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Jonathan Wood & Assoc.: Speakers Bureau; Mallinckrodt Pharmaceuticals (formeraly Therakos, Inc): Research Funding; Soligenetics: Research Funding; Evidera, Inc.: Consultancy; Guidepoint Global: Consultancy; Cell Medica Inc.: Consultancy; UT MD Anderson Cancer Center: Employment; Shape: Research Funding; USCLC Registry: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Secretary/treasurer of Item h; Hawaiian Dermatology Society: Speakers Bureau; Hemedicus: Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spatz Foundation: Research Funding; Oncoceuticals: Research Funding; Millennium (formerly Takeda): Research Funding; Allos: Research Funding; Rhizen Pharma: Research Funding; Cutaneous Lymphoma Foundation: Membership on an entity's Board of Directors or advisory committees; Cell Medica Ltd.: Honoraria; Forty Seven Inc: Membership on an entity's Board of Directors or advisory committees; Tetralogic: Research Funding. Huen:Galderma Inc: Research Funding; Glaxo Smith Kline Inc: Research Funding; Rhizen Pharmaceuticals: Research Funding; Innate Pharmaceuticals: Research Funding.
Pembrolizumab in T cell lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal