Introduction
Large granular lymphocyte (LGL) leukemia is characterized by a clonal expansion of CD3+ cytotoxic T or CD3- NK cells. Prominent clinical features include neutropenia, anemia and autoimmune-associated diseases such as rheumatoid arthritis (RA). Although the disease is usually chronic and indolent, some patients may be symptomatic and require treatment. No standard therapy has been established due to the absence of prospective clinical trials. So far, low dose methotrexate, oral cyclophosphamide, and cyclosporine represent the 3 main options for initial therapy. In 2014, we launched a prospective clinical trial comparing methotrexate to cyclophosphamide in previously-untreated patients with LGL leukemia in need of treatment.
Patients and methods
The study was designed as a multicentric, national, open label, randomized, controlled trial on two parallel groups, comparing methotrexate and cyclophosphamide. Patients were included if they had at least one of the following indications of treatment: isolated severe neutropenia (ANC <0.5x109/L) or neutropenia (ANC <1.5x109/L) with infections, anemia requiring transfusions or symptomatic anemia, associated complications such as systemic diseases or auto-immune diseases resistant to steroids and/or immunomodulating agents (colchicin, disulone, hydrochloroquine). They were randomly assigned to receive either methotrexate (10 mg/m²/w) or cyclophosphamide (100 mg/d) for 4 months. Responders at M4 continued with the same treatment until M12 (cyclophosphamide was then delivered at 50 mg/d). Non-responders at M4 were randomly assigned to receive either cyclosporine (3 mg/kg/d) or the drug which had not been administered at the first randomization (methotrexate or cyclophosphamide). Response was assessed using previously published criteria (Lamy T, Blood 123:1182, 2014). Complete response (CR) was defined as a normalization of clinical exam (disappearance of splenomegaly or associated autoimmune symptoms) and a complete normalization of blood counts. Partial response (PR) was defined as an improvement in blood counts which did not meet criteria for complete remission (e.g., ANC >0.5x109/L or decrease of transfusion requirements). Treatment failure was defined as no response or any response which did not meet the above-mentioned criteria within four months after the beginning of treatment.
To stop the trial as soon as sufficient information was collected, a sequential analysis was planned each time 20 patients were included and evaluated using the triangular test (Sébille V, Bellissant E. Fundam Clin Pharmacol. 2003;17(5):505-16). The primary endpoint was the hematological CR rate evaluated at M4 (binary endpoint). Secondary endpoints were overall response rate (ORR) at M4, M8 and M12, time to relapse. For non-responders at M4, cyclosporine was compared to the treatment which had not been administered during the first phase.
Results
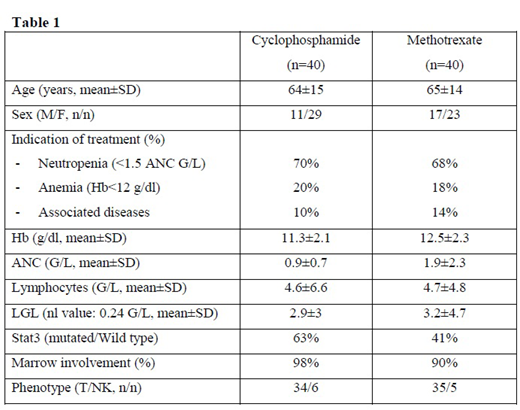
From Nov 2013 to July 2019, 99 patients met inclusion criteria among which 96 were randomized. The baseline characteristics of these patients are shown in Table1. STAT3 mutation was observed 52% of cases. After the 4th sequential analysis performed on the first 80 patients evaluable for response at M4, the sample path remained in the continuation region of the triangular test. Thus, the trial has to be continued. At M4, 13 patients were in CR (16.3%) and 29 patients were in PR (36.3%), ORR was 52.6%, 36 patients were considered as refractory and underwent a second randomization: 18 patients received cyclosporine and 17 received methotrexate or cyclophosphamide.
Conclusions
This first prospective randomized clinical trial in LGL leukemia shows that the CR after first line therapy using either methotrexate or cyclophosphamide is relatively low (< 20%). Recruitment is still ongoing to assess if there is a difference in terms of response between the two drugs. Predictive biomarkers of response will be presented at the meeting. Regarding a 52% of incidence of Stat3 mutation (higher than that previously published), Jak/Stat targeted therapy should be prospectively evaluated in this disease.
Houot:Bristol Myers Squibb: Honoraria; Merck Sharp Dohme: Honoraria. Gyan:Pfizer: Honoraria. Feugier:janssen: Honoraria, Research Funding, Speakers Bureau; abbvie: Honoraria, Research Funding, Speakers Bureau; gilead: Honoraria, Research Funding, Speakers Bureau; roche: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal