Introduction:
MCL outcomes have improved with the use of IBR; however, 33% of patients do not respond to IBR and duration of response is about 1.5 yrs (Wang ML Blood 2015). Synergy of IBR with VEN has been demonstrated (Jayappa KD Blood Advances 2017; Axelrod M Leukemia 2014). As both drugs are metabolized by CYP3A, a dose finding study, supported by a grant from AbbVie Inc., was conducted utilizing a continual re-assessment method for toxicity and efficacy in 6 dosing cohorts (see table) (NCT02419560). Here we report the optimal dose and outcomes.
Methods:
IBR-naïve MCL patients, relapsed or refractory to at least one line of therapy, were eligible if they had adequate organ function and were not at high risk for tumor lysis syndrome (TLS). Subjects were enrolled in two phases of the study. Each subject was sequentially placed into escalating dose levels. Then, subjects were allocated to a dose arm based on best response among the arms that were estimated safe based on DLT data from prior subjects. The cohort with the best response and acceptable toxicity was considered optimal (Wages NA et al, CCR 2017). DLTs were defined as any related Grade 3-5 non-hematologic or Grade 4-5 hematologic event lasting more than 2 weeks and occurring within 8 weeks of starting both VEN and IBR.
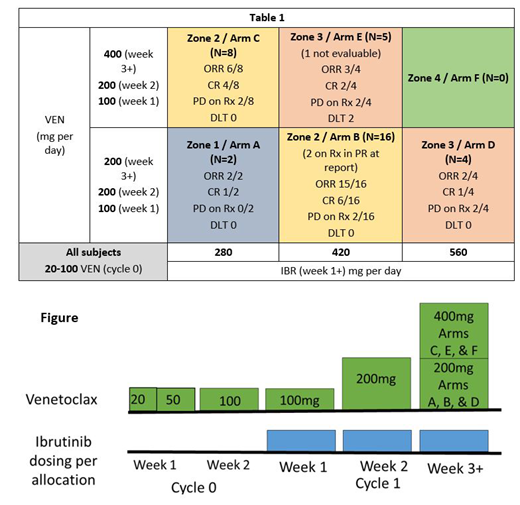
VEN was given alone for 1 week at a dose of 100mg daily. IBR was added on week 2 at the allocated dose. VEN was further titrated to its allocated dose. After one of 15 subjects experienced TLS with one 100mg dose (Davids MS JCO 2018), the study was amended to titrate VEN from 20mg to 100mg over 2 weeks before adding IBR (see figure). After amendment, the optimal dose was defined as the cohort that had 10 subjects allocated or the cohort with the max subjects allocated after 38 total subjects enrolled.
Study treatment with IBR and VEN continued for 6 months; subsequent treatment was per investigator discretion.
Results:
Enrollment began 9/2015. A total of 35 subjects were treated (15 prior to amendment and 20 after). Subjects were predominantly male (29/35) and had an average age of 62.5 yrs (range 49-81 yrs). 51.4% (n=18) were refractory to their last therapy and 42.8% (n=15) had a prior autologous transplant.
After amendment, 10 subjects were allocated to Arm B as of 2/2019, making Arm B (IBR 420mg/VEN 200mg) the optimal dose identified. No subjects were treated at the maximum dose combination as 2 DLTs occurred at dose level E (persistent Grade 4 neutropenia and Grade 3 diarrhea).
At the time of submission, ORR (CR+PR) on Arm B was 15/16 (93.8%, 90% CI:[74, 100]) with CR rate (best response) of 6/16 (37.5%, 90% CI:[18, 61]). Two subjects on Arm B are still undergoing therapy and results will be updated. No DLTs were identified in this cohort. Best ORR for all 34 evaluable subjects is 28/34 (82.4%, 90% CI:[68, 92]) with best CR rate of 14/34 (41.2%, 90% CI:[27, 57]). Five of 34 (14.7%, 90% CI:[6, 28]) subjects progressed without response and 3 additional subjects progressed after a response (all PR) (Table 1). Non-response or progression during the 6-months of study was not dependent on dose of either drug. Survival assessments are not currently mature.
The most common adverse events (AE) were neutropenia (Gr >3 n=10), thrombocytopenia (Gr>3 n=2), nausea (all Gr1 n=13), diarrhea (Gr 1 n=12; Gr>2 n=2), infections (Gr 2 n=11; Gr 3 n=3), cardiac (afib Gr 3 n=3; heart failure Gr 3 n=1) and arthralgia (Gr 1 n=9, Gr2 n=2). There were no deaths due to AEs. Five subjects had dose modifications (IBR n=1; VEN n=4, Both n=0), and 3 stopped treatment early due to AE (22.8%).
Conclusions:
In this phase I/Ib study, the combination of IBR 420mg and VEN 200mg is considered the optimal dose for relapsed MCL. Response at this level is favorable compared to historical cohorts of single agent IBR and toxicity is manageable. Two other studies have been reported using IBR 560mg and VEN 400mg in MCL, though with different dosing strategies (Tam CS NEJM 2018; Wang ML Lugano 2019). Both showed similar ORR to the current study and both had approximately 60% of subjects requiring dose reduction in at least one drug. In our study, higher dose combinations showed toxicity without an improvement in response. Progressions during the 6-months of IBR/VEN treatment occurred at all dose levels, suggesting that failure of the combination is not dose dependent but biologically driven. Dose reductions are not predicted to alter response; though survival outcomes are immature. Correlative studies are planned.
Portell:AbbVie: Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Amgen: Consultancy; Bayer: Consultancy; BeiGene: Consultancy, Research Funding; Kite: Consultancy, Research Funding; Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Roche/Genentech: Research Funding; Infinity: Research Funding. Kahl:TG Therapeutics: Consultancy; BeiGene: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy. Budde:F. Hoffmann-La Roche Ltd: Consultancy. Chen:Autolus Therapeutics: Employment. Cohen:Astra Zeneca: Research Funding; Lymphoma Research Foundation: Research Funding; ASH: Research Funding; LAM Therapeutics: Research Funding; Takeda Pharmaceuticals North America, Inc.: Research Funding; Gilead/Kite: Consultancy; Bristol-Meyers Squibb Company: Research Funding; Janssen Pharmaceuticals: Consultancy; Seattle Genetics, Inc.: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; UNUM: Research Funding; Hutchison: Research Funding. Williams:Seattle Genetics: Consultancy; Sandoz: Consultancy; Pharmacyclics: Research Funding; TG Therapeutics: Consultancy, Research Funding; Allos: Research Funding; AbbVie: Consultancy; Astra-Zeneca: Consultancy; Kite: Consultancy; Juno: Consultancy; Verastem: Consultancy; Celgene: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Venetoclax is not approved in MCL; the combination of venetoclax and ibrutinib is not approved in MCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal