Introduction: Waldenström macroglobulinemia (WM) is characterized by circulating monoclonal IgM and bone marrow infiltration with lymphoplasmacytic lymphoma (LPL) cells. Presently, there is limited data to guide prognosis or treatment options of WM based on the proportion of lymphocytes or plasma cells in the LPL population on the pathology. In this study, we aim to look at differing clinical features and outcomes of patients with WM based on the plasma cell (PC) percentage in the diagnostic bone marrow biopsy.
Methods Patients with WM seen at Mayo Clinic, Rochester, MN between January 2001 and December 2017, with a bone marrow biopsy and a corresponding serum free light chain (FLC) examination at diagnosis of WM were included. The percentage of lymphocytes and PCs in the marrow biopsy was determined based on morphology ± immunohistochemistry. On immunohistochemistry, CD138 and/or MUM-1 positivity was used to quantify PCs, whereas CD20 and/or PAX5 were used to quantify lymphocytes. Patients with ≥10% PC component in the lymphoplasmacytic (LPL) cells on bone marrow biopsy were considered to have WM with excess plasma cells (WMEPC), while those with <10% PCs were considered to have lymphocytic WM (LWM). Continuous variables were compared using Wilcoxon test and categorical variables were compared using Chi square test or Fisher's exact test. Time-to-next therapy (TTNT) was defined as the period from initiation of first line therapy to start of second line therapy or death due to any cause before second line therapy. Time-to-event analyses were conducted using Kaplan Meier method.
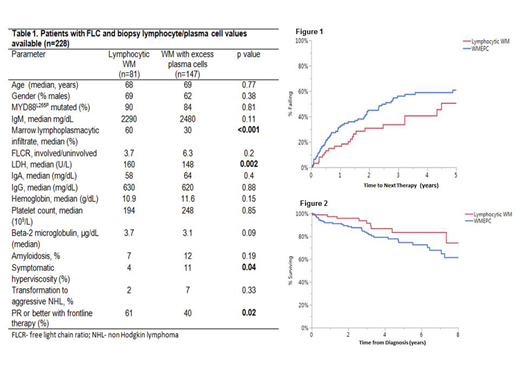
Results: Of 228 evaluable patients, 147 (64%) had ≥ 10% PCs in the initial marrow biopsy. The median follow-up was 3.4 years (95% CI: 2.3-4.3 years) for LWM and 4.3 years (95% CI: 3.4-4.8 years) for WMEPC (p=0.33). The baseline characteristics of WMEPC versus LWM are depicted in Table 1. Patients with WMEPC had lower bone marrow involvement by LPL (30%) compared to LWM (60%; p<0.001) at diagnosis, but the rate of symptomatic hyperviscosity was higher in WMEPC during the disease course (Table 1). The TTNT was shorter for WMEPC [2.6 years (95% CI: 1.8-4.5 years)] compared to LWM [4.5 years (95% CI 3.2-8.2)], although it did not reach statistical significance (p=0.09, Figure 1). There was a trend towards shorter OS for WMEPC [Not reached (95% CI 7.2- NR)] compared to LWM [10.7 years (95% CI 7.3 -12.1 years); p=0.28], Figure 2. Of the 228 patients, 79% (n=182) had data available for systemic therapy. The rate of PR or better with frontline therapy was higher in rates in LWM compared to WMEPC (61 % vs 42% p=0.02, Table 1). Among WMEPC cohort, patients receiving either a proteasome inhibitor (PI)-based or an immunomodulatory (IMiD)-based combination as frontline therapy (n=16) had higher rates of partial response or better (68%) compared to non-PI/non-IMiD based therapy (n=114), p=0.04.
Conclusion: WM with excess plasma cells at diagnosis is associated with a trend towards shorter TTNT and OS compared to WM with <10% plasma cells in the lymphoplasmacytic population at diagnosis. Our preliminary data shows higher rates of partial response or better with the use of PI/IMiD based therapy, but the small cohort size limits the strength of this finding which needs to be validated externally.
Ansell:Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Affimed: Research Funding. Gertz:Spectrum: Honoraria, Research Funding; Janssen: Honoraria; Celgene: Honoraria; Prothena: Honoraria; Alnylam: Honoraria; Ionis: Honoraria. Dingli:alexion: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Rigel: Consultancy; Karyopharm: Research Funding. Nowakowski:Selvita: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding; MorphoSys: Consultancy, Research Funding; Genentech, Inc.: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Curis: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Dispenzieri:Alnylam: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Janssen: Consultancy; Intellia: Consultancy; Akcea: Consultancy. Lacy:Celgene: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Kapoor:Janssen: Research Funding; Cellectar: Consultancy; Glaxo Smith Kline: Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal