Background:
Patients with follicular lymphoma (FL) have a heterogenous prognosis. Recently a simple score, the PRIMA-PI, was developed based on the PRIMA clinical trial (Bachy et al Blood. 2018). With only two factors (beta-2-microglobulin level > 3 mg/L and bone marrow involvement), this index was at least as discriminatory as FLIPI on the training and validation cohorts. The validity of the PRIMA-PI was confirmed on Czech and German FL cohorts and a patient group from the Nordic Lymphoma Group. However, further validation is needed to confirm the use of PRIMA-PI in place of FLIPI for prognostic assessment. Indeed, in the era of new chemo-free treatments, it seems important to challenge the potency of traditional prognostic factors and scores. Recently, rituximab combined with lenalidomide (R2) was compared to conventional immunochemotherapy (R-chemo) in the phase III RELEVANCE trial. The aim of our study was to validate PRIMA-PI in the RELEVANCE trial cohort and compare its performance with FLIPI (Solal-Celigny et al. Blood. 2004) and FLIPI2 (Federico et al. JCO. 2009). A secondary objective was to evaluate potential differences in terms of prognostic bio-clinical parameters between the R2 and R-chemo arms.
Methods:
All patients with available data for FLIPI, FLIPI2, and PRIMA-PI from the intention to treat population of the RELEVANCE study were included in the analysis. PFS according to each prognostic score were assessed in the total population and by treatment arms. Data were not mature enough to compare OS distributions. Performance metrics (log-rank p value and Net Reclassification Improvement [NRI]) were calculated for each group to assess concordance and discriminating ability of each score.
Results:
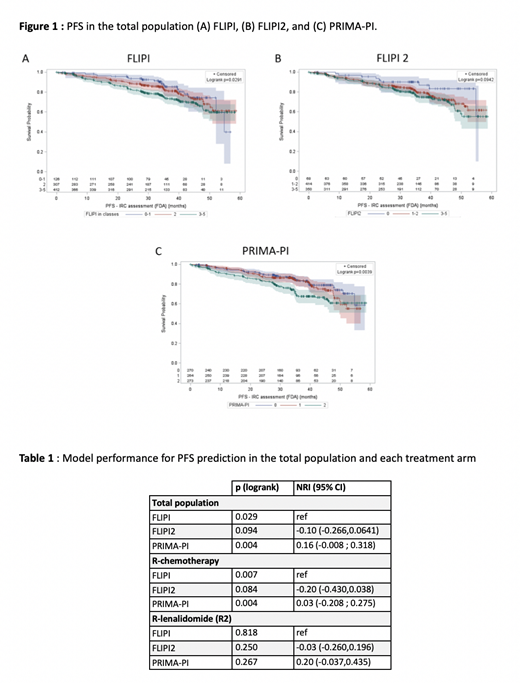
Median follow-up time for the study was 38 months. Overall, 846 RELEVANCE patients were included in the analysis. Data were available for 845 patients for FLIPI score assessment, 832 for FLIPI2 and 807 for PRIMA-PI. Group repartition according to the FLIPI and the FLIPI2 were largely imbalanced compared with PRIMA-PI. FLIPI classified very few patients in the low risk group (15% LR) while 49% of the patients were at high risk (HR), and 36% were at intermediate risk (IR). Similarly, FLIPI2 risk categories were as follow: 8% LR, 50% IR, and 42% HR. On the contrary, PRIMA-PI divided the study population into three equal groups (33%, 33% and 34%). In the total population, FLIPI and PRIMA-PI were predictive of PFS (p=0.029 and p=0.004, respectively); FLIPI2 showed poor performance (p=0.094). PFS curves based on each score are shown in Figure 1. NRI index indicated that the PRIMA-PI yielded analogous segregation for PFS with FLIPI (NRI 0.16; 95% CI: -0.008, 0.318; Table 1). In the R-chemo arm, both FLIPI and PRIMA-PI could isolate different prognostic groups for PFS, whereas FLIPI2 could not. Conversely, none of the indices were able to significantly discriminate outcomes for patients treated with R2. Interestingly, analysis showed that some usual prognostic factors, especially those likely to reflect tumor burden such as beta-2 microglobulin and LDH, were not predictive for PFS in the R2 arm. In contrast, low albumin (<40 g/L) and low hemoglobin (<120 g/L) levels were significantly associated with worse PFS in the R2 arm (p=0.001, and p=0.023), but not in the R-chemo arm.
Conclusion:
For patients with FL treated upfront with immunochemotherapy, the PRIMA-PI is a valid scoring system that allows to segregate patients as efficiently as the FLIPI while using only two factors. These results confirm that the PRIMA-PI could substitute for the FLIPI for patients treated with upfront R-chemo. Athough our data have to be interpreted in light of the short median follow-up time, they also suggest that other clinical/biological parameters might be considered and new prognostic indexes established for patients treated with R2. Considering possible mechanisms of action of lenalidomide, prognostic factors related to the underlying patient's immunological status might be more predictive.
Fowler:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; ABBVIE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Bachy:Roche: Consultancy; Janssen Cilag: Honoraria; Gilead Science: Honoraria; Amgen: Honoraria; Roche: Honoraria; Janssen Cilag: Other: Travel, accomodation, Expense. Feugier:abbvie: Honoraria, Research Funding, Speakers Bureau; roche: Honoraria, Research Funding, Speakers Bureau; janssen: Honoraria, Research Funding, Speakers Bureau; gilead: Honoraria, Research Funding, Speakers Bureau. Tilly:roche: Membership on an entity's Board of Directors or advisory committees; servier: Honoraria; merck: Honoraria; Gilead: Honoraria; Janssen: Honoraria; BMS: Honoraria; Karyopharm: Consultancy; Astra-Zeneca: Consultancy; Roche: Consultancy; Celgene: Consultancy, Research Funding. Palomba:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Noble Insights: Consultancy; Evelo: Other: Immediate family member, Equity Ownership; MSK (IP for Juno and Seres): Other: Immediate Family Member, Patents & Royalties - describe: intellectual property rights ; Hemedicus: Other: Immediate Family Member, Speakers Bureau ; Merck & Co Inc.: Other: Immediate Family Member, Consultancy (includes expert testimony); Seres Therapeutics: Other: Immediate Family Member, Equity Ownership and Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees. Libby:Akcea: Consultancy; Alnylam: Consultancy; Abbvie: Consultancy; Pharmacyclics and Janssen: Consultancy. Casasnovas:Merck Sharp and Dohme: Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses. Flinn:TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; AbbVie, Seattle Genetics, TG Therapeutics, Verastem: Consultancy; Acerta Pharma, Agios, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Genentech, Gilead Sciences, Incyte, Infinity Pharmaceuticals, Janssen, Karyopharm Therapeutics, Kite Pharma, Novartis, Pharmacyclics, Portola Pharmaceuticals: Research Funding. Haioun:Novartis: Honoraria; Janssen: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria; Servier: Honoraria; Takeda: Honoraria; Miltenyi: Honoraria; Gilead: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Bartlett:Pharmacyclics: Research Funding; Pfizer: Research Funding; Affimed: Research Funding; Celgene: Research Funding; Millenium: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Forty Seven: Research Funding; Bristol-Myers Squibb: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Genenetech: Research Funding; Gilead: Research Funding; Immune Design: Research Funding; Janssen: Research Funding. Bouabdallah:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Brice:BMS: Honoraria; Millennium Takeda: Research Funding; Takeda France: Consultancy, Honoraria. Ribrag:Nanostring: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Research Funding; ArgenX: Research Funding; Roche: Other: Travel, accommodations, and expenses ; BMS: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses ; MSD: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; AZ: Membership on an entity's Board of Directors or advisory committees. Le Gouill:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Roche-Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Martín:Teva: Research Funding; Gilead: Consultancy, Honoraria; Kiowa Kirin: Consultancy; Roche: Consultancy, Honoraria, Other: Travel Expenses; iQone: Consultancy; Servier: Honoraria, Other: Travel Expenses; Janssen: Honoraria, Other: Travel Expenses, Research Funding; Celgene: Consultancy, Honoraria, Other: Travel Expenses, Research Funding. Lopez-Guillermo:Roche: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Research Funding. Larouche:Bayer; Gilead Sciences; Merck; Roche: Research Funding. Ando:Eisai: Research Funding. Maria:Janssen Cilag: Consultancy, Other: Travel support; Gilead Sciences: Other: Travel support, Research Funding; Abbvie: Consultancy, Other: Travel support; Celgene: Consultancy; Roche: Consultancy, Other: Travel support. André:Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers-Squibb: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel grants, Research Funding; Amgen: Other: Travel grants, Research Funding; Johnson & Johnson: Research Funding; Takeda Millenium: Research Funding; Chugai: Research Funding; Celgene: Other: Travel grants, Research Funding. Sehn:Merck: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Janssen-Ortho: Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria. Tobinai:Daiichi Sankyo: Consultancy, Honoraria; Eisai: Honoraria, Research Funding; Verastem: Honoraria; Mundi Pharma: Consultancy, Honoraria, Research Funding; Zenyaku Kogyo: Consultancy, Honoraria; Yakult: Honoraria; Janssen Pharmaceutical: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; HUYA Bioscience: Consultancy, Honoraria; Ono Pharmaceutical: Consultancy, Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; AbbVie: Research Funding; Solasia: Honoraria; Meiji Seika: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceutical: Consultancy, Honoraria, Research Funding. Cartron:Roche, Celgene: Consultancy; Sanofi, Gilead, Janssen, Roche, Celgene: Honoraria. Delarue:Celgene Corporation: Employment, Equity Ownership. Czuczman:Celgene Corporation: Employment, Equity Ownership. Salles:BMS: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis, Servier, AbbVie, Karyopharm, Kite, MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Amgen: Honoraria, Other: Educational events; Epizyme: Consultancy, Honoraria; Roche, Janssen, Gilead, Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Morschhauser:Bayer: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal