Background:
The effectiveness of anti-tumour T and NK cells in malignancy is mitigated by the engagement of the immune check point pathway with PD-1/PDL-1 interaction.1,2 Immune manipulation with PD-1 inhibition, in combination with rituximab, can increase T cell anti-tumour effect and enhance NK cell antibody dependent cell cytotoxicity (ADCC), with this combination proving efficacious in rituximab-refractory follicular lymphoma (FL).3 The concept of 'priming' the immune system with nivolumab, a PD-1 inhibitor, prior to tumour-directed therapy has rationale and evidence, but the safety of this approach in previously untreated FL is not described.4 We are conducting a phase II Simon's two stage study to assess the feasibility and safety of combination nivolumab and rituximab in these patients and present the pre-planned interim analysis results.
Methods:
The 1st FLOR study (NCT03245021) is an ongoing open-label, multi-centre, phase 2 study of nivolumab and rituximab in patients with previously untreated stage III-IV grade 1-3A FL requiring systemic therapy. Eligible patients are ECOG ≤ 2 and do not require urgent debulking therapy. All patients receive induction nivolumab 240mg intravenously (IV) every 2 weeks for 4 cycles. Patients achieving complete response (CR) based on PET/CT Lugano criteria receive a further 4 cycles of 240mg IV nivolumab monotherapy then maintenance nivolumab 480mg IV every 4 weeks for 12 cycles. Patients achieving less than CR received 240mg nivolumab plus 375mg/m2 IV rituximab every 2 weeks for 4 cycles. Those achieving a response at the end of 8 cycles of nivolumab and rituximab continue maintenance nivolumab 480mg IV every 4 weeks for 12 cycles PLUS rituximab 375mg/m2 every 12 weeks for 8 cycles. PET/CT assessment is performed at baseline, after 4 cycles of nivolumab, after 8 cycles of nivolumab +/-rituximab and prior to maintenance cycle 6. The primary end point is safety, with a pre-planned analysis after the first 19 patients completed induction treatment with 8 cycles of nivolumab +/- rituximab. Secondary endpoints included response rates and time to treatment failure (TTF). The planned total study population is 39 patients, with a Simon's two-stage design. We report the results of the pre-planned interim analysis.
Results:
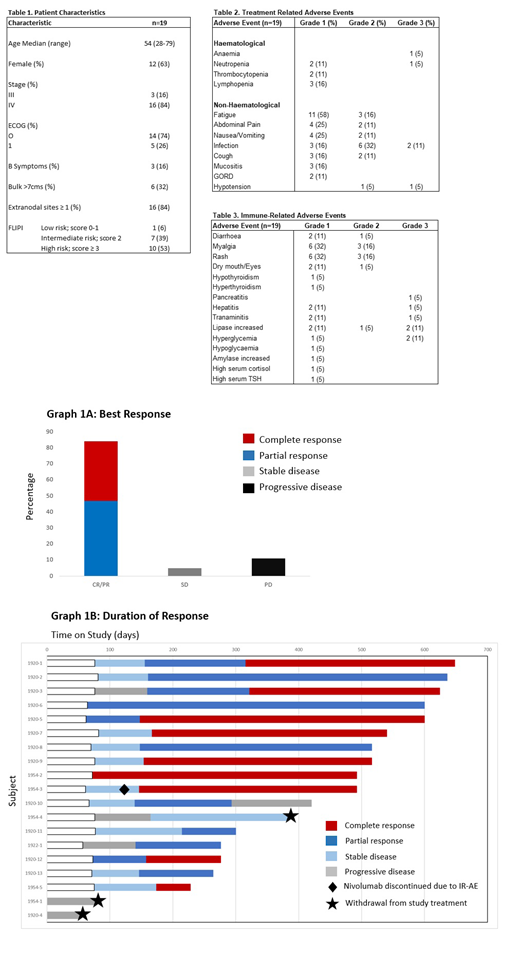
Baseline characteristics are summarised in Table 1. Treatment-related adverse events (AEs) were predominantly grade 1-2 with fatigue (74%), infection (59%), nausea/vomiting (36%) and abdominal pain (36%) being the most common (Table 2). Immune-related (IR) AEs were reported in 79% of all patients. Grade 3-4 IRAEs were uncommon and included; pancreatitis plus hepatitis (1 patient); transaminitis (n=1), hyperglycaemia (n=2) and asymptomatic lipase increase (n=2) (Table 3).
Median follow-up was 17 months. The overall response rate (ORR) was 84% (16/19) with 47% (9/19) achieving CR, 37% (7/19) partial response (PR), 5% (1/19) stable disease and 11% (2/19) progressive disease (PD) as best response (Figure 1A and 1B). Median time to CR was 5 months. Median TTF was 3.4 months. Three pts (16%) discontinued study treatment; 2 due to early disease progression (1 with suspicion of transformation in cycle 2) and 1 due to development of constitutional symptoms with stable disease.
Conclusion:
In this planned interim analysis, the combination of nivolumab and rituximab in treatment naive follicular lymphoma is associated with a favourable toxicity profile and high overall and complete response rates potentially providing patients an alternative to traditional chemotherapy.
Acknowledgements:
Bristol-myers Squibb provided funding and nivolumab for this study.
References:
1. Salmaninejad A, et al. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol.
2. Ferris RL, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev.
3. Nastoupil LJ, et al. Response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: Interim results of an on open-label, phase II study. J Clin Oncol.
4. Park SE, et al. Increased Response Rates to Salvage Chemotherapy Administered after PD-1/PD-L1 Inhibitors in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol.
Chong:Novartis: Research Funding; Hutchison Medipharma: Research Funding; Pharmacyclics: Research Funding; Bayer: Research Funding; Merck Serono: Research Funding; BMS: Research Funding. Gilbertson:Roche: Speakers Bureau; Roche: Honoraria. Grigg:Janssen: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel. Ritchie:Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; BMS: Research Funding; Takeda: Research Funding; Beigene: Research Funding; Imago: Research Funding; Novartis: Honoraria; Sanofi: Honoraria. Koldej:NanoString Technologies: Other: Travel grant. Manos:Novo Nordisk Pharmaceuticals: Other: Travel; Janssen: Honoraria. Hawkes:Astra Zeneca: Research Funding; BMS: Research Funding, Speakers Bureau; Merck KgA: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Mundi pharma: Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Speakers Bureau; Merck Sharpe & Dohme: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Takeda: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Nivolumab is a fully human IgG4 monoclonal antibody against programmed cell death 1 (PD-1). The PD-1 pathway is an immune system checkpoint that may be exploited by tumour cells to escape immune surveillance. By blocking the binding of the PD-1 receptor to the PD-1 and PD- 2 ligands, nivolumab reactivates tumour-specific cytotoxic T-lymphocytes in the tumour microenvironment to restimulate anti-tumour immunity.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal