
SAA is a life-threatening hematopoietic stem cell disorder that is often treated with bone marrow transplantation (BMT) to reconstitute hematopoiesis and eliminate clonality. Our group has shown that PTCy after related HLA-haplo BMT can facilitate engraftment and yield low rates of relapsed and refractory (R/R) SAA. Given the risk of relapse or secondary clonal disease following immunosuppressive therapy (IST) is > 50% at five years, we decided to implement this treatment paradigm in untreated SAA. Here, we review the haplo outcomes in SAA, with an update in R/R SAA patients and new results in treatment-naïve (TN) SAA patients using haplo donors and PTCy.
Patients were eligible with SAA. Fanconi anemia and short telomere patients were excluded. R/R patients had ≥ one course of IST. The conditioning regimen included standard published haplo backbone of antithymocyte globulin, low dose CY, and total body irradiation (TBI). All R/R patients received 200cGy. The TBI dose for the TN cohort was modified after 3 graft failures (GF) in initial 7 patients treated at 200cGy; thus, the last 8 TN patients received 400cGy. After the marrow graft was infused on day 0, cyclophosphamide 50mg/kg/day IV on days +3,+4 post-transplant was administered. All received mycophenolate mofetil beginning day +5 through 35 and tacrolimus day +5 to 365. Engraftment definitions include: absolute neutrophil count >1.0 x10E9/L for three consecutive days; days from last red blood cell transfusion for hemoglobin and platelet count >50,000 for 7 days without transfusion.
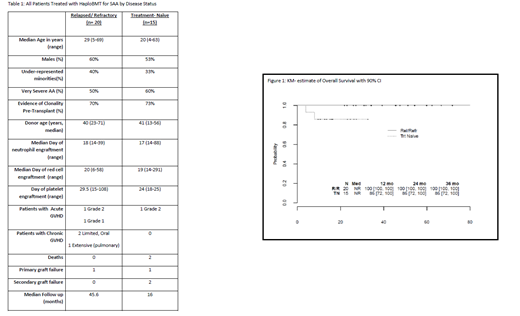
Thirty-five SAA patients received haplo BMT: 20 R/R and 15 TN. The median follow-up is 31 months (90% CI: 26.0, 45.4). The median age was 25 (range, 4-69) years, with 23 patients > age 20 and 57% were males. The median related haplo donor age was 34 years (range, 19-57), including three non-first degree relatives. Table 1 shows data by cohort. Median time to neutrophil recovery was 17 days (range 15-88), to red cell recovery 23 days (range 6-58) and to platelet recovery 28 days (range 15-108). At the time of BMT, 25 of 35 patients had evidence of clonality (mostly PNH clones). Four of 35 patients (11.4%) (95% CI: 3.2, 26.7%) experienced GF. There was one GF out of 20 patients in the R/R group (5%) (95% CI: 0.1, 24.9%) and 3 of 15 in the TN group 20% (95% CI: 4.3,48.1%, p= 0.20). OS for all patients (Figure 1) is 94% (90% CI: 88,100%) at one and two years, 100% in the R/R group and 86% (90% CI: 72, 100%) in the TN group. The cumulative incidence of grade II-IV aGVHD at day 100 is 9% (90% CI: 1, 17%). The cumulative incidence of chronic GVHD at 2 years is 9% (90% CI: 1,17%). All R/R patients are alive and well, and fully engrafted with 100% donor chimerism; patient with primary GF is 100% chimeric after 2nd transplant from different haplo donor. One patient has extensive cGVHD. Of the first 7 TN patients who received 200cGy TBI, 4 are fully engrafted and well; of the 3 with GF, 2 died from infection and one is now 100% chimeric using second haplo donor. After increasing the TBI dose to 400cGy, the subsequent 8 patients are alive with full donor chimerism.
Nonmyeloablative haplo BMT with PTCy represents a potential cure in SAA, with all 20 R/R currently alive, disease-free (including eradication of clonal diseases), and no evidence of active GVHD. Extending this approach to TN patients was associated with higher GF rates. Perhaps this is not surprising given that the potential beneficial effect of front-line IST on engraftment is absent. The increase in TBI dose to 400cGy in TN patients appears to achieve more durable engraftment without early greater toxicity (infection, delayed engraftment, GVHD); more patients and longer follow up are required to understand rates of fertility and other survivorship issues. Alternative donor BMT (including haplo donors) should be offered to all SAA patients with R/R SAA who are fit enough to tolerate non-myeloablative conditioning. A potential advantage of haplo donors over unrelated donors is that the transplant center has full control and oversight of allograft quality, which can be especially important with the use of the preferred allograft source for SAA, bone marrow. Non-myeloablative haplo BMT in TN SAA could lead to a paradigm-shift in the field, such that essentially all children and fit adults can proceed quickly to a relatively safe, curative BMT.
DeZern:Astex Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy. Cooke:Jazz pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal