Background: Acute myeloid leukemia (AML) is a hematological malignancy characterized by the autonomous growth of immature myeloid cells with impaired differentiation and maturation. Cytokines are low-molecular-weight proteins that play a basic and fundamental role in communication within the immune system. Cytokines induce various effects such as differentiation, proliferation, hematopoiesis, and inflammation of target cells. AML is also closely associated with cytokine networks in terms of proliferation, apoptosis, and differentiation of leukemic cells. Cytokines produced by Th1 involved in cell-mediated immunity are called Th1 cytokines. Th1 cytokine includes TNF-α and IL-2. Several studies have reported that TNF-α is highly expressed in leukemia cells with AML patients. Other studies have also reported that high serum level of TNF-α of AML patients is associated with poor survival outcome. However, the association between Th1 cytokine polymorphisms: TNF-α -857C/T and IL-2-330T/G and the pathogenesis of AML is unclear. Therefore, we investigated the role of these polymorphisms in AML.
Materials and Methods: This study included 101 patients with AML [male/female, 56/45; age, 15-86 years; median age, 58 years; MRC classification favorable (n = 38), intermediate (n =56), and adverse (n = 7)] and 202 healthy race-matched controls. All participants provided written informed consent. This study was approved by the Institutional Review Board of Gunma University Hospital. Genotyping was performed by the polymerase chain reaction (PCR)-restriction fragment length polymorphism method. Genotype and allele frequency were compared between patient group and control group by χ2-test. Clinical features were compared using Student's t and χ2 tests. Overall survival (OS) and leukemia free survival (LFS) were calculated using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Analyses were performed using the SPSS software package ver. 25 (IBM, Armonk, NY, USA). P < 0.05 was considered to represent statistical significance.
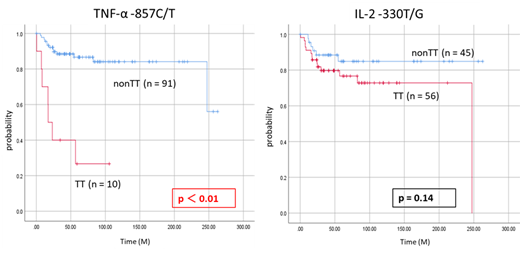
Results: TNF-α -857 C/T nonCC genotype (higher producer type) increases the risk of AML (AML vs. controls = 39.6% vs. 28.2%, OR = 1.67, 95% CI = 1.01-2.75, p = 0.045). Moreover, the frequency of TNF-α -857 C/T T allele (higher producer type) was higher in AML patients compared to controls (AML vs. controls = 24.8% vs. 16.8%, OR = 1.625, 95%CI = 1.078-2.451 p = 0.02). There was no significant difference between AML patients and controls in genotype and allele frequencies of IL-2 -330 T/G. In the analysis of clinical features, the average platelet count was significantly lower in TNF-α -857 C/T TT genotype (higher producer type) (TT vs. nonTT = 2.4±1.4 vs. 4.4±5.9, p < 0.01). TT genotype (higher producer type) was also significantly higher in frequency of MRC classification adverse (TT vs. nonTT = 30.0% vs. 4.4%, p = 0.02) and history of tumor (TT vs. nonTT = 30.0% vs. 6.6%. p =0.04). Moreover, in survival time analysis, patients with TNF-α -857 C/T TT genotype (higher producer type) had significantly shortened OS compared with patients with nonTT genotype (lower producer type) (TT vs. nonTT = 17.2 months vs not reached, p < 0.01). Patients with TT genotype (high producer type) also experienced significantly shortened LFS (TT vs. nonTT = 24.0 months vs not reached, p = 0.04). Furthermore, multivariate analysis of OS revealed TNF-α -857 C/T TT genotype (higher producer type) as an independent prognostic factor (HR = 3.01, 95% CI = 1.04-8.69, p = 0.04), like age and white blood cell count.
Conclusion: These results suggest that TNF-α-857 C/T T allele (higher producer type) increases the risk of AML. Furthermore, TNF-α-857 C/T TT genotype (higher producer type) affects the poor prognosis. Therefore, these data suggest the new role of TNF-α polymorphism in AML leukemogenesis.
Handa:Ono: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal