Introduction: Although induction chemotherapy results in a complete remission (CR) in ~70% of newly diagnosed AML patients, post-remission therapies are needed to eliminate minimal residual disease (MRD) and prevent relapse. Consolidation chemotherapy, either as definitive therapy or bridge to bone marrow transplantation (BMT), is currently the most common form of post-remission therapy. Yet, our understanding of its impact on MRD remains limited. In this study, we investigated the effects of consolidation chemotherapy on molecular MRD (mMRD) burden using ultra-deep next generation sequencing (NGS) and correlated treatment response with disease characteristics and survival outcomes in AML patients.
Patients and Methods: 91 newly diagnosed AML patients who achieved CR following standard induction chemotherapy were evaluated. Targeted conventional NGS using a 54-gene panel was performed on whole blood (PB) or bone marrow samples collected at diagnosis. PB samples were collected during remission at two consecutive time points (T1 and T2), before and after 1 (n=79) or 2 (n=12) cycles of consolidation chemotherapy, for each patient. To detect mMRD, we used a custom 37-gene hybrid-capture panel and error-corrected NGS based on the duplex sequencing approach with a variant allele frequency (VAF) detection limit of ~1x10-4. For 10 patients, we also performed duplex sequencing analysis on their relapsed samples.
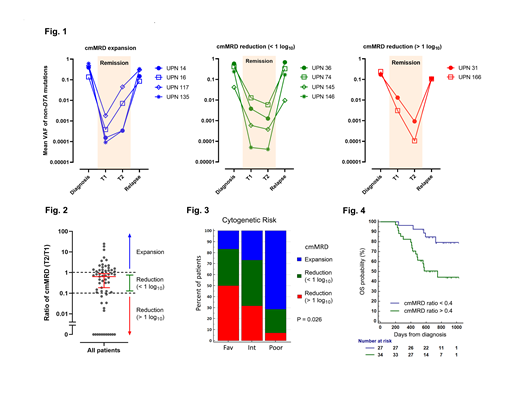
Results: NGS of the diagnostic samples identified a total of 298 putative oncogenic mutations in 92% (n=84) of the 91 patients. Ninety percent of these mutations (n=267) were trackable by the custom hybrid-capture panel. Duplex sequencing detected persistence of 56% (n=149) of the trackable mutations in T1 samples; 34% (n=50) of which were clonal hematopoiesis-associated DTA mutations (those involving DNMT3A, TET2, or ASXL1), and the remaining 66% (n=99) were non-DTA mutations. Analysis of T2 samples showed that consolidation chemotherapy reduced the VAF of non-DTA mutations by a median of 73% and cleared 27% (n=27) of them at T2. In contrast, the burden of DTA mutations increased by 0.5% (P < 0.0001 by Mann-Whitney test), and only 2% (n=1) of the mutations was cleared (P = 0.0001 by Fisher's exact test). These findings are consistent with prior studies demonstrating that non-DTA mutations are more reliable markers of leukemic burden than DTA mutations.
To study the impact of consolidation chemotherapy at the level of individual patients, the mean VAF of all persistent non-DTA mutations was calculated for each sample and used as a composite measure of mMRD burden (henceforth referred to as "cmMRD"). Analysis of the 10 patients with relapsed samples showed that cmMRD levels tracked well with achievement of remission and disease progression (Fig. 1). In the subset of patients with persistent non-DTA mutations at T1 (n=61), consolidation chemotherapy decreased cmMRD levels by a median of 36% at T2. However, we observed high interpatient variability (Fig. 2); 36% (n=22) of the patients experienced an increase in cmMRD burden after consolidation chemotherapy, and 36% (n=22) had less than a 1 log reduction. Only 28% (n=17) of the patients achieved a log reduction of greater than 1. The likelihood and magnitude of cmMRD response were significantly associated with cytogenetic risk (P = 0.026 by 3x3 Chi-square test; Fig. 3). The proportion of patients with favorable, intermediate, and poor-risk cytogenetics who experienced cmMRD expansion was 17%, 27%, and 71%, respectively. Consistent with these findings, a suboptimal response (defined as cmMRD ratio [T2/T1] > 0.4) was associated with inferior overall survival (HR = 3.29, P = 0.007 by log-rank test; Fig. 4).
Conclusions: Our analysis showed that mMRD response to consolidation chemotherapy was highly variable among patients. Although consolidation chemotherapy was effective in deepening the remission for a subset of patients, it failed to lower MRD levels for a substantial proportion of patients, especially those with poor risk cytogenetics. These findings challenge the practice of using consolidation chemotherapy to achieve a deeper remission prior to BMT for high-risk patients and indicate that the opposite outcome may occur instead. NGS-based monitoring of mMRD can potentially be used to distinguish between patients who can remain on consolidation chemotherapy as definitive therapy and those who require a switch in post-remission therapy.
Gupta:Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Research Funding. Maze:Pfizer Inc: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. McNamara:Novartis Pharmaceutical Canada Inc.: Consultancy. Minden:Trillium Therapetuics: Other: licensing agreement. Schimmer:Medivir Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Consultancy; Novartis Pharmaceuticals: Consultancy; Otsuka Pharmaceuticals: Consultancy. Schuh:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva Canada Innovation: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Yee:Novartis, Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas, Celgene, Otsuka, Shire, Takeda: Membership on an entity's Board of Directors or advisory committees; Agensys, Astex, Hoffman La Roche, MedImmune, Merck, Millenium, Roche/Genentech: Research Funding. Bratman:SVB: Other: is co-inventor of a patent relating to circulating tumor DNA detection technology, which has been licensed to Roche Molecular Diagnostics.. Chan:Agios: Honoraria; AbbVie Pharmaceuticals: Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal