【Background】Recently, FLT3 inhibitors are approved for FLT3 mutated AML patients and other FLT3 inhibitors are also in clinical development. Since the target selectivity and the inhibitory activity of FLT3 inhibitors are varied, it is required to establish a therapeutic strategy in consideration of their characteristics. Most FLT3 mutated AML cells co-express wild type (Wt)-FLT3, and we previously demonstrated that FLT3 ligand (FL) stimulation attenuated the inhibitory effect of FLT3 inhibitors through the activation of Wt-FLT3 in Wt- and ITD-FLT3 co-expressing cells; however, little is known about this inhibitory mechanism in recently developed FLT3 inhibitors. In this study, we aimed to clarify the efficacy and characteristics of four FLT3 inhibitors including the impact of FL on AML cell lines and primary patients cells, and to identify biomarkers for drug selection and prediction of their efficacy.
【Methods】We examined the growth inhibitory effects of midostaurin, quizartinib, gilteritinib and FF-10101 at various concentrations of FL in ITD-FLT3 expressing 32D cells, Wt- and ITD-FLT3 co-expressing 32D cells,and FL and Wt-FLT3 co-expressing 32D cells. The inhibitory effects of these four FLT3 inhibitors were also examined in 87 primary AML cells with or without FLT3 mutationin vitro, and the correlation between their efficacy and clinical and molecular characteristics including genetic alterations and FLT3-ITD allelic ratio (ITD-AR) were investigated. Moreover, characteristics of residual AML cells after the treatment with FLT3 inhibitors were also examined in patient-derived xenografts (PDX) -AML model.
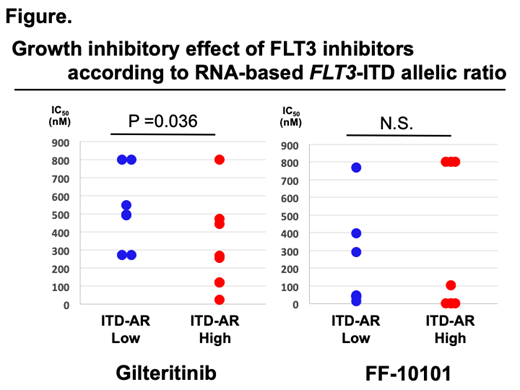
【Results】The inhibitory effect of FLT3 inhibitors was significantly impaired by FL stimulation dose dependently in all inhibitors except for midostaurin in Wt- and ITD-FLT3 co-expressing 32D cells. In FL and Wt-FLT3 co-expressing cells, GI50 value of gilteritinib was higher than that in ITD-FLT3 solo-expressing cells, and the similar tendency was observed with quizartinib. We examined the Growth inhibitory effects of these inhibitors in 33 FLT3 mutated and 54 FLT3 un-mutated primary AML cells. In primary cells, midostaurin showed lower selectivity to FLT3 mutation compared with other inhibitors. Subsequently, the correlation between the efficacy of FLT3 inhibitors and ITD-AR was examined. RNA or DNA based ITD-AR was not related to the growth inhibitory effect of FLT3 inhibitors except for gilteritinib in FLT3-ITD mutated patient cells; the GI50 value of gilteritinib in AML cells with RNA based ITD-AR-low were significantly higher than those in RNA based ITD-AR-high (P=0.036) (Figure).Moreover, FLT3-ITD cells with mutated NPM1 tend to have higher GI50values for all of FLT3 inhibitors, irrespective of ITD-AR. In AML-PDX treated with quizartinib or gilteritinib, FLT3-ITD-AR in residual AML cells was lower than that of non-treated cells, suggesting that co-expression level of Wt-FLT3 is related to the response to FLT3 inhibitors in vivo.
【Conclusions】FL affected the efficacy of FLT3 inhibitors in Wt- and ITD-FLT3 co-expressing cells, and the inhibitory effects on ITD-FLT3 and FL-Wt-FLT3 pathway were different among FLT3 inhibitors. Furthermore, NPM1 mutation and RNA based ITD-AR might be predictive biomarkers for the efficacy of FLT3 inhibitors in FLT3-ITD positive AML. The appropriate therapeutic strategy based on the characteristics of each inhibitor is necessary.
Ishikawa:Bristol-Myers Squibb: Honoraria; Abbvie GK.: Honoraria; Kyowa Hakko Kirin Co., Ltd.: Honoraria; Celgene Co., Ltd.: Honoraria. Goto:Celgene Co., Ltd.: Honoraria; Novartis Pharma Co., Ltd.: Honoraria; JCR Pharmaceuticals Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria. Ozawa:Pfizer Japan Inc.: Honoraria; Novartis: Honoraria; Kyowa-Hakko Kirin: Honoraria; Astellas Pharma Inc.: Honoraria. Kiyoi:Zenyaku Kogyo Co., Ltd.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; Astellas Pharma Inc.: Honoraria, Research Funding; Otsuka Pharmaceutical Co.,Ltd.: Research Funding; FUJIFILM Corporation: Research Funding; Pfizer Japan Inc.: Honoraria; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Daiichi Sankyo Co., Ltd: Research Funding; Bristol-Myers Squibb: Research Funding; Perseus Proteomics Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal