
Introduction: The phase 3 ADMIRAL trial demonstrated that gilteritinib, a novel, potent, oral FLT3 inhibitor, significantly prolonged overall survival and resulted in higher remission rates compared with salvage chemotherapy in patients with FLT3-mutation-positive (FLT3mut+) relapsed/refractory (R/R) acute myeloid leukemia (AML; Perl AE, et al. AACR 2019), even in the presence of common co-occurring AML mutations (DNMT3A, NPM1, and WT1) (Levis MJ, et al. J Clin Oncol. 2019;37[suppl 15]:7000). However, as with other FLT3 inhibitors, patients often develop resistance after an initial response to gilteritinib. Evidence suggests that expansion of leukemic clones containing mutations in Ras/MAPK pathway genes NRAS and KRAS mediates secondary resistance to gilteritinib in patients with FLT3mut+ R/R AML, and confirms that cells with Ras/MAPK pathway mutations are FLT3mut+ (McMahon CM, et al. Cancer Discov. 2019; doi: 10.1158/2159-8290). We evaluated emerging mutations in patients who relapsed while receiving gilteritinib therapy in the ADMIRAL trial.
Methods: Blood or bone marrow samples were available for 361 patients at baseline (97.3% of the intention-to-treat population [N=371]) and for 40 patients who relapsed on gilteritinib treatment. Samples were analyzed by next-generation sequencing using the Archer Core Myeloid Panel. Data were analyzed using Archer Analysis software; the variant allele frequency (VAF) cutoff was ≥2.7%.
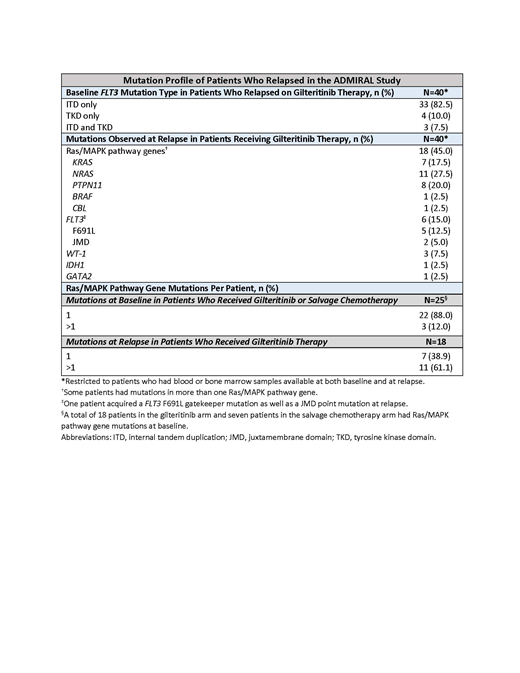
Results: Of 371 patients enrolled in the ADMIRAL trial, 247 were assigned to 120-mg/day gilteritinib and 75 (30.5%) relapsed during the study. Most relapses (n=72/75; 96.0%) occurred ≤4 weeks from the last gilteritinib dose. Forty patients who had samples available at baseline also had samples at relapse for comparison. No samples were available from patients who relapsed on chemotherapy. At relapse, 27/40 patients (67.5%) had new mutations, including mutations in Ras/MAPK pathway genes (n=18), FLT3 (n=6), WT1 (n=3), IDH1 (n=1), and GATA2 (n=1) (Table). Thirteen patients (32.5%) had no new mutations.
Of the 18 patients with Ras/MAPK pathway gene mutations at relapse, 11 (61.1%) had >1 new mutation at relapse (range, 2-6). The most frequently mutated Ras/MAPK pathway gene was NRAS (n=11). Patients were also assessed for Ras/MAPK gene pathway mutations prior to gilteritinib therapy. Among all FLT3mut+ patients analyzed for co-mutated genes at baseline (n=361), 25 (6.9%) had Ras/MAPK pathway gene mutations detected (gilteritinib, n=18; salvage chemotherapy, n=7; median VAF, 13% [range, 3.4%-50%]). In contrast to the 12.0% of patients (n=3/25) who had >1 Ras/MAPK pathway gene mutation at baseline, 61.1% (n=11/18) had >1 Ras/MAPK pathway gene mutation at relapse.
Notably, a considerable number of gilteritinib-treated patients who had Ras/MAPK pathway gene mutations at baseline achieved remission: the rate of CRc (ie, composite complete remission: complete remission [CR] or CR with incomplete hematologic/platelet recovery) was 38.9% (n=7/18); the rate of CR/CRh (ie, CR or CR with partial hematologic recovery) was 27.8% (n=5/18). Six patients acquired new FLT3 mutations at relapse. Five of these six patients acquired a F691L gatekeeper mutation; one of these five patients also acquired a FLT3 juxtamembrane domain point mutation. Of the three patients who acquired a WT1 mutation at relapse, one also acquired a FLT3 F691L gatekeeper mutation. The acquisition of Ras/MAPK pathway gene mutations and FLT3 F691L gatekeeper mutations at relapse was mutually exclusive.
Conclusions: In patients with FLT3mut+ R/R AML who relapsed on gilteritinib therapy, Ras/MAPK pathway gene mutations and FLT3 F691L gatekeeper mutations were the most common mutational events. The presence of a Ras/MAPK pathway gene mutation at baseline did not preclude benefit from gilteritinib therapy, possibly due to fewer Ras/MAPK pathway gene mutations per patient at baseline than at relapse. The acquisition of multiple Ras/MAPK pathway gene mutations at relapse likely mediates continued engagement of Ras/MAPK signaling in patients with FLT3mut+ R/R AML receiving gilteritinib. The frequency of emergent FLT3 F691 gatekeeper mutations at relapse in patients who received 120-mg/day gilteritinib in the ADMIRAL study was similar to that observed in relapsed patients who received 20- to 450-mg/day gilteritinib (Levis MJ, et al. Blood. 2017;130[suppl 1]:2705).
Smith:Revolution Medicines: Research Funding; Astellas Pharma: Research Funding; fujiFilm: Research Funding; Abbvie: Research Funding. Levis:Astellas: Consultancy, Research Funding; FUJIFILM: Consultancy, Research Funding; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Daiichi Sankyo Inc: Consultancy, Honoraria. Perl:Astellas: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of trial data., Research Funding; BioMed Valley Discoveries: Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Other, Research Funding; Arog: Consultancy, Other: Non-financial support included travel costs for advisory board meetings.; AbbVie: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Actinium Pharmaceuticals: Consultancy, Honoraria, Other: Clinical Advisory Board member, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Non-financial support included travel costs for advisory board meetings.; Jazz: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; NewLink Genetics: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Takeda: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Bayer: Research Funding; FujiFilm: Research Funding; Novartis: Honoraria, Other: Advisory board, Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of the data., Research Funding. Martinelli:Daiichi Sankyo: Consultancy, Honoraria; Roche: Consultancy, Other: trial grant; Ariad: Consultancy, Other: trial grant; Janssen: Consultancy, Other: trial grant; Amgen: Consultancy, Other: trial grant; Pfizer: Consultancy, Other: trial grant; Abbvie: Consultancy, Honoraria, Other: trial grant; Celgene: Consultancy, Honoraria, Other: trial grant; Novartis: Consultancy, Other: trial grant; Incyte: Consultancy, Other: trial grant. Berman:Astellas: Membership on an entity's Board of Directors or advisory committees, Research Funding. Montesinos:Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: Research support; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Teva: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Research support, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Baer:Abbvie: Research Funding; Astellas: Research Funding; Al Therapeutics: Research Funding; Forma: Research Funding; Incyte: Research Funding; Kite: Research Funding; Takeda: Research Funding. Larson:Celgene: Consultancy; Agios: Consultancy; Novartis: Honoraria, Other: Contracts for clinical trials. Yokoyama:Astellas: Other: Travel expenses. Recher:Incyte: Honoraria; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Macrogenics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Yoon:MSD: Consultancy; Kyowa Hako Kirin: Research Funding; Janssen: Consultancy; Genentech, Inc.: Research Funding; Amgen: Consultancy, Honoraria; Yuhan Pharma: Research Funding; Novartis: Consultancy, Honoraria. Hill:Astellas: Employment; Ligacept, LLC.: Other: Stock, Patents & Royalties. Rosales:Astellas: Employment. Bahceci:Astellas: Employment, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal