Rationale:
Inotuzumab ozagomicin (IO) has been linked to an increased incidence of veno-occlusive disease (VOD) and liver alterations. Most VOD events occurred during hematopoietic stem cell (HSCT) transplantation after IO therapy. We have previously described that the measurement of liver stiffness can anticipate the diagnosis of VOD in the context of HSCT. The mechanisms underlying the increased risk of VOD and liver damage in patients receiving IO are not well understood; in the pathogenesis endothelial damage, ozagomicin release and on-target off-tumor effects may be involved. Here, we aimed to assess the effects of IO on the changes of liver, vascular and biochemistry parameters.
Methods:
Intensive monitoring of the liver was incorporated into the standard of care of patients who received IO for relapsed or refractory (R / R) acute lymphoblastic leukemia (ALL). Upper abdomen ultrasound with Doppler was performed at baseline and at the end of therapy; liver stiffness measurement (LSM) by Fibroscan® (Echosens, Paris, France) at every IO course or at every IO infusion. With the exception of ursodeoxycholic acid, the patients did not receive prophylaxis for VOD. Data was collected after anonymous aggregation, in accordance with GCP and Helsinki declaration. Results are reported as median with interquartile ranges (IQR).
Results:
At data cut-off, 1st Apr 2019, 16 patient received baseline assessment and at least a post-IO assessment in our monitoring program. In our patent set, median age was 44.5 (IQR 30.7 - 64.0); 12/16 (75 %) patients relapsed after the last treatment and 4/16 (25 %) patients were refractory to the last treatment; patients received a median of 3 (IQR 2 - 3.7) lines before IO; 6/16 (37.5 %) patients undergone HSCT before IO, of which a patient had 1st and 2nd HSCT before IO; 5/16 (31.25 %) undergone HSCT after IO therapy (no patients had second HSCT after IO). Patients received a median of 2 (IQR 2.0 - 3.7) IO administration according to the schedule of the phase 3 trial. The median duration of the therapy was 61.5 days (IQR 43.2 - 114.0) and median progression-free survival in our population was 278.0 days (95% C.I. 264.0 - 292.0).
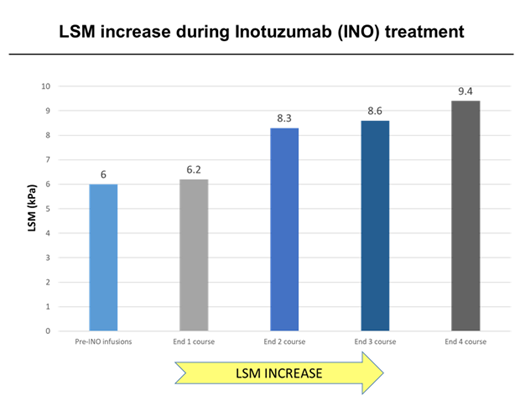
In our patient set, we performed 113 biochemistry determination, 30 liver ultrasounds with Doppler and 116 LSM examination. One patient received a liver biopsy. Among the biochemical exams (AST, ALT, GGT and alkaline phosphatase) only the AST values significantly increased after 1st course of IO (from the median value of 21 U/L to 53 U/L after course 3). Liver ultrasound with Doppler revealed portal hypertension signs in half of the patients during IO monitoring program. Among these patients 7/16 (44%), 3/16 (17%), 5/16 (33.3%) and 3/16 (17%) showed splenomegaly, recanalization of the paraumbilical vein, dilatation of portal vein and ascites, respectively. Median LSM significantly increased from a baseline value of 6 kPa to 7.8 kPa after last post-IO assessment (p-value<0.01). The median increase of LSM values on the baseline after course 1 of IO was 3.3% (0%- 4.9%), after course 2 was 38.3% (26.4% - 45.2%) after course 3 was 43.3% (35.4% - 48.6%) and after course 4 was 56.7 (45.8% - 60.1%), see figure. Eight of the 16 patients (50%) showed an increase in LSM with values comparable to fibrosis higher than 2 (> 7.1 kPa). With a median follow up of 387.5 days (IQR 182.8-524.5) we observed one VOD event (7%); the VOD was graded severe and occurred after HSCT post-IO.
Conclusions:
Our clinical experience represents the first step to better understand the IO-related liver alterations, as we described the frequency and relevance of quantitative markers. Most of the patients in our set developed ultrasound and/or elastography alteration during IO therapy. Furthermore, these alterations do not seem to correlate with biochemistry. Even if most of the patients had sub-clinical vascular and parenchymal alterations of the liver portal-hypertension related, VOD incidence in our set is comparable with literature. Long-term follow-up results are expected to test whether alterations return or evolve over time. Stratifying the tailored risk liver complications with prospective non-invasive and marker-driven strategies in term of IO dosing and HSCT timing could be a great benefit for patients.
* FR and GM contributed to this manuscript equally
#AC and CP contributed to this manuscript equally
Martinelli:Roche: Consultancy; BMS: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; ARIAD: Consultancy. Cavo:janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau; bms: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; novartis: Honoraria; takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel accommodations, Speakers Bureau. Papayannidis:Amgen: Honoraria; Novartis: Honoraria; Teva: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; Shire: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal