
Background: Because infections are a major cause of morbidity and mortality after AML induction chemotherapy, patients typically remain hospitalized for monitoring and rapid antimicrobial therapy until hematopoietic recovery. With declining early mortality and improved oral antimicrobials, interest in moving post-induction care to the outpatient setting has emerged. In the 5-year period since completing a prospective phase 2 trial evaluating an Early Hospital Discharge (EHD) strategy, EHD following AML-like induction chemotherapy has become routine at our institution. In recent retrospective analyses, we found >80% of EHD patients required hospital readmission, primarily for neutropenic fever. Still, the EHD strategy was safe and reduced healthcare resource utilization, and EHD patients spent >70% of their post-chemotherapy time as outpatients. Here, we investigated differences in the pattern of infectious complications between patients managed as outpatients following induction chemotherapy and those who remain hospitalized until hematopoietic recovery.
Methods: We retrospectively identified all adults ≥18 years with untreated AML/high-grade myeloid neoplasms (≥10% blasts in blood/ bone marrow) who started intensive induction chemotherapy ("7+3" or a regimen of similar/higher intensity) at our institution from 8/1/2014-7/31/2018. Patients were considered "EHD" if they were discharged from the hospital <72 hours from completing chemotherapy (as done in our phase 2 study); the remaining patients were considered inpatient "controls". The study period began on the day of discharge (EHD group) or at the completion of chemotherapy (control group) and ended with count recovery, hospital discharge (control group), death, receipt of more chemotherapy, or at 42 days. Table 1 describes baseline variables collected. Outcomes of interest were organism and site of infections, days of IV antimicrobial use, days in intensive care unit (ICU), and survival. Bacterial infections were either culture-documented or probable based on clinical exam/imaging. Fungal infections were proven or probable based on EORTC/MSG Working Group Criteria. Microbiological documentation was required for viral infections.
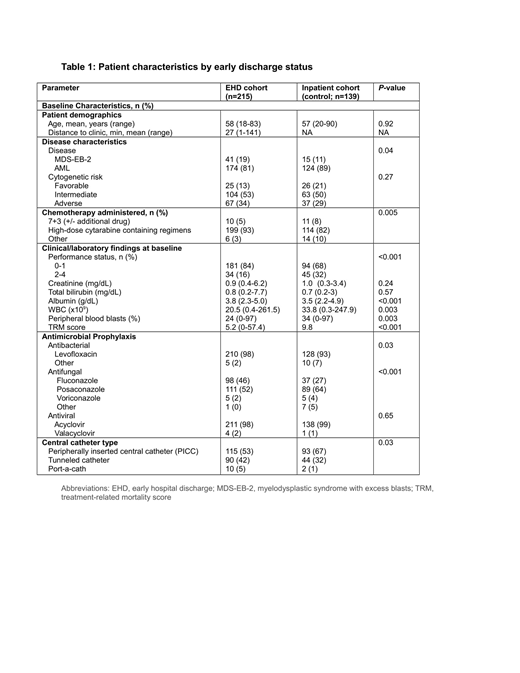
Results: We identified 354 inductions that met our criteria: 215 (61%) in the EHD group and 139 (39%) in the control group (baseline characteristics in Table 1). For antimicrobial prophylaxis, more EHD patients than controls used levofloxacin vs. other agents (98% vs. 93%; p=0.03) and controls more likely used posaconazole vs. EHD patients (64% vs. 52%; p<0.001). EHD patients more likely had a tunneled central venous catheter (42% vs. 32%; p=0.03) than a peripherally inserted central catheter (53% vs 67%; p=0.03). 53% of EHD patients vs. 39% of controls developed ≥1 infection during the study period (p=0.01). There were no differences between groups in type of organism identified. We then assessed whether the higher infection rate in EHD patients led to inferior outcomes (Table 2). There were no differences in ICU admission (7% vs. 10%; p=0.44) or early death (4% vs. 7%; p=0.24) between EHD and controls. Despite a higher rate of infections, EHD patients spent fewer days on IV antimicrobials. For the whole cohort, however, patients with ≥1 infection more likely required ICU care than those without infection (14% vs 4%, p<0.001) but there was no difference in early death rates between patients who developed an infection and those who did not. Development of gram positive bacteremia was associated with risk of ICU admission, a trend towards early death and a longer hospital stay. Development of fungal infection was also associated with a longer hospital stay (12.7 vs 6.4 days) but not risk of ICU admission or early death. There was no association between type of prophylactic antifungal agent and subsequent diagnosis of fungal infection, between catheter type and risk of bacteremia, or between housing distance to our institution and infection risk (EHD group).
Conclusion: An EHD care strategy following AML-like induction chemotherapy is associated with a higher rate of infection but not an increased risk for ICU stay or early death. Central catheter type, specifics of antimicrobial prophylaxis, and distance to our institution were not associated with infection risk in the EHD group. Further investigation to elucidate the risk factors for the increased rate of infection in the EHD group is warranted.
Halpern:Pfizer Pharmaceuticals: Research Funding; Bayer Pharmaceuticals: Research Funding. Othus:Glycomimetics: Other: Data Safety and Monitoring Committee; Celgene: Other: Data Safety and Monitoring Committee. Buckley:CTI Biopharma: Employment. Percival:Pfizer Inc.: Research Funding; Nohla Therapeutics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Becker:The France Foundation: Honoraria; Accordant Health Services/Caremark: Consultancy; AbbVie, Amgen, Bristol-Myers Squibb, Glycomimetics, Invivoscribe, JW Pharmaceuticals, Novartis, Trovagene: Research Funding. Scott:Incyte: Consultancy; Novartis: Consultancy; Agios: Consultancy; Celgene: Consultancy. Oehler:Pfizer Inc.: Research Funding; Blueprint Medicines: Consultancy; NCCN: Consultancy. Gernsheimer:Bioverativ: Consultancy; Novartis: Honoraria; Dova pharmaceuticals: Consultancy; Shionogi: Consultancy; Rigel: Consultancy; Fuji film: Consultancy; Amgen: Consultancy, Honoraria; Cellphire: Consultancy. Orozco:Actinium Pharmaceuticals: Research Funding. Cassaday:Seattle Genetics: Other: Spouse's disclosure: employment, stock and other ownership interests; Amgen: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Kite/Gilead: Research Funding. Shustov:Seattle Genetics, Inc.: Research Funding. Hartley:Pfizer Inc.: Employment, Equity Ownership. Welch:Pfizer Inc: Employment, Equity Ownership. Walter:Seattle Genetics: Research Funding; Jazz Pharmaceuticals: Consultancy; Amphivena Therapeutics: Consultancy, Equity Ownership; Agios: Consultancy; Amgen: Consultancy; Aptevo Therapeutics: Consultancy, Research Funding; Argenx BVBA: Consultancy; Astellas: Consultancy; BioLineRx: Consultancy; BiVictriX: Consultancy; Boehringer Ingelheim: Consultancy; Daiichi Sankyo: Consultancy; New Link Genetics: Consultancy; Pfizer: Consultancy, Research Funding; Kite Pharma: Consultancy; Covagen: Consultancy; Boston Biomedical: Consultancy; Race Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal