Background:
The use of midostaurin in combination with standard induction chemotherapy has been shown to improve the outcomes in patients with newly diagnosed FLT3 mutated (FLT3m) AML (Stone et al) and allogeneic transplant is often recommended in first remission. While likely to achieve remission with standard induction, the majority of these patients will relapse and die of their disease. Gilteritinib, a potent FLT3 inhibitor, has been approved for use in patients with relapsed or refractory FLT3 mutated AML. In Phase I/II studies, when used as part of induction therapy, gilteritinib has shown promising CR rates. We have designed a trial comparing the outcomes of induction therapy with gilteritinib to those with midostaurin. The hypothesis is that gilteritinib, when added to induction chemotherapy, will produce more FLT3m negative composite complete remission (CRc).
The Ratify trial combined midostaurin with daunorubicin 60 mg/m2/d X 3 days and cytarabine X 7 days Daunorubicin 90 mg/m2/d has however been shown to be superior during induction in patients with newly diagnosed FLT3 ITD mutated AML (Luskin et al, Burnett et al) While improved survival is the ultimate goal of any leukemia regimen, it is known that AML patients who proceed to allogeneic transplant with measurable residual disease (MRD) positive disease fare worse than those with MRD negative disease. As such, our goal is to increase the likelihood of an MRD negative state after induction.
Study Design
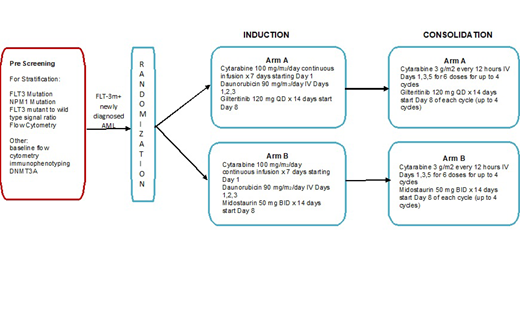
This is a randomized, open-label, multicenter phase II study comparing gilteritinib to midostaurin in combination with cytarabine 100 mg/m2 and daunorubicin 90 mg/m2 in newly diagnosed FLT3 mutated AML patients ages 18-65. Central confirmation of FLT3 mutation and other pretreatment studies are required. In order to identify patients without delay, all patients with suspected AML will be able to be prescreened without enrollment to the randomized treatment trial.
Patients will be stratified according to TKD vs. ITD FLT3 mutation. Patients with FLT3-ITD mutation will undergo further stratification with NPM1 mutation status (positive vs. negative) and FLT3 ITD to FLT3 wild type signal ratio (high [≥ 0.5] vs. low [<0.5]). Patients will be randomized to receive standard induction treatment (cytarabine and daunorubicin) with either gilteritinib or midostaurin. Mandatory bone marrow samples are required after Induction and Consolidation. Patients who achieve CR or complete remission with incomplete hematologic recovery (CRi) will be assessed for MRD and be eligible at count recovery to go on to receive standard consolidation treatment of high-dose cytarabine with the study drug received during induction (gilteritinib or midostaurin). Patients may proceed to allogeneic transplant after induction or after 0-4 cycles of chemotherapy consolidation and may participate in BMT CTN 1506.
Key Eligibility:
Newly diagnosed FLT3m AML
Primary Objective
To evaluate the FLT3m negative (evaluated by polymerase chain reaction [PCR] and capillary electrophoresis) composite complete response (CRc) (includes CR or CRi)rate of patients with FLT3 mutated AML who receive gilteritinib compared to those who receive midostaurin in addition to standard therapy with cytarabine and daunorubicin during induction.
Sample Size and Statistical design
The accrual goal of this study will be 179 patients. Patients who achieve remission will be followed for survival and relapse. With an allocation ratio of 1:1 to the two arms, the study will have 80% power to detect an improvement of 20% in the FLT3 mutation negative CRc rate in the gilteritinib arm (i.e. from 40% to 60%) at the one-sided significance level of 0.05 based on Fisher's exact test.
This study is conducted through PrECOG and is funded by Astellas Pharma. The study is slated to open in the summer of 2019 at 30 US sites.
References:
Stone R.M., Mandrekar S.J., Sanford B.L., et al. N Engl J Med 2017; 377:454-464
Luskin MR, Lee JW, Fernandez HF et al. Blood 2016 :blood-2015-07-657403; doi: https://doi.org/10.1182/blood-2015-07-657403
Burnett AK, Russell NH, Hills RK et al. Blood 2016; Blood 2016 128:449-452; doi: https://doi.org/10.1182/blood-2016-04-712091
Luger:Genetech: Research Funding; Jazz: Honoraria; Celgene: Research Funding; Pfizer: Honoraria; Kura: Research Funding; Onconova: Research Funding; Agios: Honoraria; Ariad: Research Funding; Biosight: Research Funding; Seattle Genetics: Research Funding; Cyslacel: Research Funding; Daichi Sankyo: Honoraria. Loghavi:MDACC: Employment; GLG Consultants: Consultancy; AlphaSights: Consultancy. Lazarus:Genentech: Speakers Bureau; Pluristem Therapeutics, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Honoraria; CSL Behring: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Actinium Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Biosight: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Teva Pharmaceuticals: Speakers Bureau. Rowe:BioSight: Consultancy. Tallman:Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; ADC Therapeutics: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellerant: Research Funding; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties. Pratz:Astellas Pharma: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Boston Biomedical: Consultancy.
In this study gilteritinib is being studies in newly diagnosed AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal