Introduction:
Short telomere syndromes (STS) are accelerated aging syndromes affecting hematopoietic, pulmonary, hepatobiliary and/or immunological systems. Clinical assessment of age-appropriate telomere length (TL) is performed using flow cytometry & fluorescence in-situ hybridization (flowFISH). Screening for germline variants in STS-related genes is guided by flowFISH-determined centile categories of TL, with screening recommended for TL <1st centile or 1-10th centile in lymphocytes (L) or granulocytes (G). However, the utility of genetic testing for patients with TL >10th centile and integration of clinical phenotype with flowFISH data in predictive algorithms is currently unclear.
Methods:
FlowFISH testing was done at reference laboratories in Vancouver (Repeat Diagnostics; Canada) & Johns Hopkins University (JHU, USA). Salient clinical features were pre-determined as, personal history of premature hair greying (onset at age < 30 years), idiopathic pulmonary fibrosis (IPF) or IPF/emphysema overlap (in smokers), cryptogenic cirrhosis or NRH, unexplained cytopenias &/or immunodeficiency, & family history of the above (in >1 1st or 2nd degree relatives). Clinical likelihood score (CLS) was assigned as low (1), intermediate (int, 2) or high (>2), based on the number of aforementioned clinical features present prior to flowFISH testing. Genetic testing was performed using either an in-house or commercial bone marrow failure-specific next generation sequencing (NGS) panel or whole exome sequencing (WES), and data for known variants affecting telomerase or telomeric function (TERT, TERC, DKC1, TINF2, NHP2, NOP10, TCAB1, NAF1, & RTEL1) was recorded.
Results:
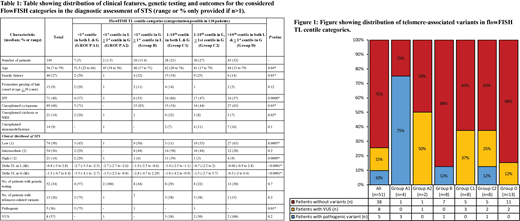
One hundred forty-nine patients at our institution underwent TL assessment at Repeat diagnostics (n=38) and JHU (n=111) laboratories, respectively. Median age was 56 (range: 7-79) years; 88 (59%) being males. Significant family history was present in 40 (27%) patients, while premature greying of hair was present in 13 (9%) patients. Organ-specific clinical features included unexplained cytopenias (n=89, 60%) IPF (n=71, 48%), cryptogenic cirrhosis or NRH (n=21, 14%), & unexplained immunodeficiency (n=14, 9%). CLS stratification included low (n=74, 50%), int (n=54, 36%), & high (n=21, 14%), with higher CLS significantly correlating with lower delta TL for L (p=0.0005) but not G (p=0.3). Genetic testing was performed in 51 (35%) patients (NGS-51, WES-1) among which 13 (26%) patients had a telomere-associated variant; 5 (10%) pathogenic (pv, all TERT). CLS alone was unable to predict likelihood of finding a telomere-associated variant (p=0.4). Based on age-appropriate centile categorization of L & G TL (information for both available in 134 patients), patients were stratified into six groups (table 1).
TL <1st centile in L: This group was further divided into two groups; TL<1st centile in both L & G [A1, n=7, CLS low-3 (43%), int-2 (29%), & high-2 (29%)] and TL <1st centile in L and 1-50th centile in G (A2, n=2, CLS low & high) patients. Among the 4 (57%) patients who underwent genetic (NGS-3, WES-1) testing, 3 (75%) had TERTpv in A1 subgroup and 1 A2 subgroup patient had a VUS in TERT.
TL <1st centile in G, 1-10th centile in L (n=18): This group included 9 (50%) low, 8 (44%) int and 1 (6) high CLS patients, of which only 1 of 8 NGS-tested patients had TERTpv.
TL 1-10th centile in L or G: This group was divided into; 1-10th centile in both L & G [C1, n=28, CLS low-3 (11%), int-14 (50%), high-11(39%)] of whom 8 (22%) underwent NGS with no pathogenic variants but 3 VUS in RTEL1, NAF1 & PARN genes, and 1-10th centile in L, >1-90th centile in G [C2, n=36, CLS low-19 (53%), 16 (44%), 1 (3%)] of whom 8 (22%) underwent NGS with 1 TERTpv and 2 VUS in TINF2
TL >10th centile in L & 1-90th centile in G (n=43, 32%): CLS stratification in this group included 27 (63%) low, 12 (28%) int, 4 (9%) high. NGS testing was done in 13 (30%) patients [CLS low-9(69%), int 2(15%), high 2 (15%)], of whom only 2 (15%) had VUS in TINF2 and TERT gene, but no pathogenic variants (figure 1).
Conclusion:
Our study demonstrates the importance of using a flowFISH assay based predictive algorithm to screen patients with suspected STS for telomere-related genetic alternations, in comparison to a clinical likelihood score. We also demonstrate a limited role for genetic testing in patients with lymphocyte TL >10th centile, regardless of the clinical likelihood score.
Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal