Introduction:
Whole blood is regaining popularity in resuscitation after hemorrhage. The platelet storage lesion that has been observed in apheresis platelets also exists in whole blood, where platelet count and aggregation response decline rapidly after collection and during storage in the blood bank. Platelet microaggregates form in unfiltered whole blood, resulting in relatively large but unstable thrombus formation in a flow model over collagen. We hypothesized that the presence of fibrinogen activates platelets within the whole blood unit and predisposes them toward premature aggregate formation.
Methods:
Fresh whole blood (WB) was collected in citrate tubes (4.3%, 10.9 mM) from healthy donors (n=4) according to an institutionally approved standard operating procedure. Blood was centrifuged at 200-g to collect the platelet-rich plasma (PRP). The remaining red blood cell (RBC) fraction was centrifuged at 2000-g to pellet RBCs which were washed with HEPES-buffered saline and repelleted; platelet-poor plasma (PPP) was saved. The PRP fraction was centrifuged with 1 µM prostacyclin at 700-g, and the platelet pellet was washed with Tyrode's buffer, repelleted, and resuspended in either fibrinogen-deficient plasma (Fg-DP) or PPP. Platelets were combined with washed RBCs to generate a reassembled whole blood sample containing 150,000 platelets/µl and a hematocrit of 40%. Samples were added to minibags and stored at 4 °C for 14 days.
On days of testing (0, 3, 7, 14), 1 ml aliquots were stained with 1 µM calcein-AM for 30 min at 37 °C and run on the BioFlux 1000 microfluidics platform with arterial flow rates generating a shear rate of 980 s-1 over a collagen surface. Fluorescence microscopy images were captured and quantified over 10 min to observe platelet adhesion to the surface. Complete blood count (CBC) was measured on each aliquot.
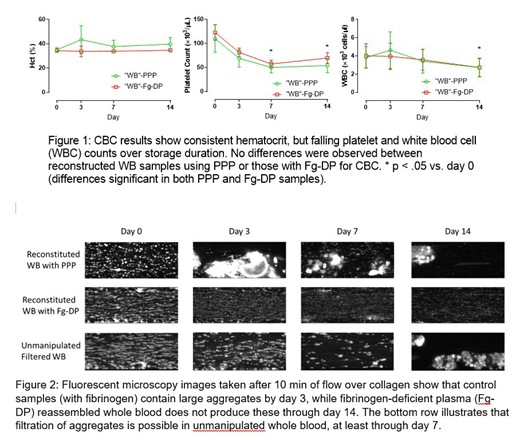
In a follow-up study, unmanipulated, stored whole blood was passed through a standard transfusion filter (200 µm pores) to determine the effects of removing microaggregates prior to microfluidics flow testing at each time point.
Results:
CBC results are in Figure 1, illustrating the decline of platelet count over storage as previously observed.
Figure 2 shows representative images captured at the end of the 10 min flow period. In samples containing normal plasma, thrombus formation is impaired by day 3 of storage with aggregate structures appearing immediately in the microscopy images. These aggregates attach and release periodically over the course of flow, an effect that persisted through day 14. In samples reconstructed with Fg-DP, no microaggregates were observed during storage, although the density and size of thrombi diminished over time. In filtered whole blood samples, thrombus formation on days 3 and 7 more closely resembled the Day 0 fresh samples, but aggregates appeared on day 14 despite filtration.
Discussion:
WB contains all of the elements required for resuscitation after hemorrhage, however the platelet storage lesion may affect its function. The interaction of platelets and fibrinogen in a relatively static environment results in activation and aggregation of platelets. In apheresis units, studies have shown that using a platelet additive solution to dilute fibrinogen results in better platelet performance for longer periods of storage. Further study is required to determine the clinical significance of aggregate formation and whether mitigation strategies should be explored.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal